217794
DL-3-Aminoisobutyric acid
98%, for peptide synthesis
Synonym(s):
α-Methyl-β-alanine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
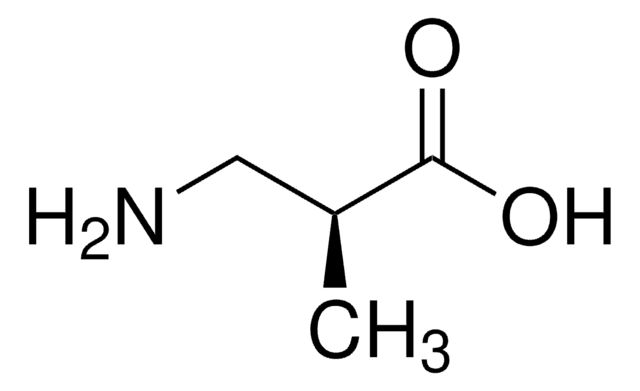
Linear Formula:
NH2CH2CH(CH3)COOH
CAS Number:
Molecular Weight:
103.12
Beilstein:
1720958
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
DL-3-Aminoisobutyric acid, 98%
Assay
98%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
179-182 °C (lit.)
application(s)
peptide synthesis
SMILES string
CC(CN)C(O)=O
InChI
1S/C4H9NO2/c1-3(2-5)4(6)7/h3H,2,5H2,1H3,(H,6,7)
InChI key
QCHPKSFMDHPSNR-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Anja Kittel et al.
Biochemical and biophysical research communications, 430(1), 84-89 (2012-11-17)
Elevated plasma concentrations of the asymmetric (ADMA) and symmetric (SDMA) dimethylarginine have repeatedly been linked to adverse cardiovascular clinical outcomes. Both dimethylarginines can be degraded by alanine-glyoxylate aminotransferase 2 (Agxt2), which is also the key enzyme responsible for the degradation
Karima Begriche et al.
Fundamental & clinical pharmacology, 24(3), 269-282 (2009-09-09)
Beta-aminoisobutyric acid (BAIBA) is a catabolite of thymine and antiretroviral thymine analogues AZT and d4T. We recently discovered that this beta-amino acid is able to enhance fatty acid oxidation and reduce body weight in mice through an increased production of
Ts Enkhjargal et al.
Rinsho byori. The Japanese journal of clinical pathology, 52(1), 17-21 (2004-02-19)
The level of beta-aminoisobutyric acid (beta-AIB), a thymine catabolite, has been measured in urine samples of 160 healthy individuals, 28 patients with renal, 27 patients with cardiovascular and 27 patients with hematological diseases and of 36 tumor patients. No significant
Nailija Khajievna Spitsyna et al.
Journal of physiological anthropology and applied human science, 24(4), 345-349 (2005-08-05)
A complex anthropological survey based on population-genetic methods and a study of a wide spectrum of genetic systems (43 alleles from 17 independent loci) was undertaken among 450 Buryat women of post-reproductive age. The results obtained showed the influence of
M Jóźwik et al.
Placenta, 19(7), 531-538 (1998-10-20)
Placental uptake and transport of three nonmetabolizable amino acids with different reactivities for transport systems were studied in sheep under normal physiologic conditions. Methylaminoisobutyric acid (MeAIB), which has specific affinity for the sodium-dependent A system transporters, demonstrated placental concentrative uptake
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service