150215
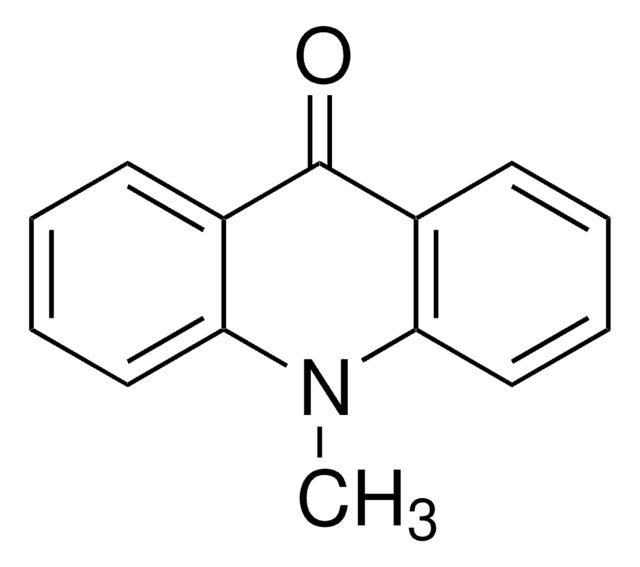
9(10H)-Acridanone
99%
Synonym(s):
Acridone, 9,10-Dihydro-9-oxoacridine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H9NO
CAS Number:
Molecular Weight:
195.22
Beilstein:
7104
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
>300 °C (lit.)
λmax
380 nm
399 nm (2nd)
SMILES string
O=C1c2ccccc2Nc3ccccc13
InChI
1S/C13H9NO/c15-13-9-5-1-3-7-11(9)14-12-8-4-2-6-10(12)13/h1-8H,(H,14,15)
InChI key
FZEYVTFCMJSGMP-UHFFFAOYSA-N
Gene Information
human ... ABCB1(5243)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
9(10H)-Acridanone (acridone) was used in the preparation of methyl 9,10-dihydro-9-oxoacridine-10-pentanoate.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dadi Chen et al.
Biotechnology for biofuels, 13, 130-130 (2020-07-24)
Considerable interest has been expressed in the development of anaerobic digestion (AD) of straw to solve the environmental problems caused by the dumping and burning of straw and to generate clean energy. However, the poor biodegradability of straw and the
Giuseppe Manfroni et al.
Journal of medicinal chemistry, 52(10), 3354-3365 (2009-04-25)
We report the synthesis and structure-activity relationship (SAR) of a large series of acridones and acridone-fragment derivatives designed on the basis of the selective antihepatitis C virus (HCV) activity shown by acridone 2, previously studied as a potential antibovine viral
Vishal P Zambre et al.
Journal of molecular graphics & modelling, 29(2), 229-239 (2010-08-10)
G-quadruplex structures of DNA represent a potentially useful target for anticancer drugs. Telomerase enzyme, involved in immortalization of cancer cells is inhibited by stabilization of G-quadruplex at the ends of chromosomes. Anthraquinone and acridone derivatives are promising G-quadruplex ligands as
Jatinder Kaur et al.
Chemical communications (Cambridge, England), 47(15), 4472-4474 (2011-03-08)
Acridones carrying an appropriate substituent at N-10 showed significant fluorescence changes on interacting with ATP in HEPES buffer at pH 7.2. The selectivity and sufficient binding of these probes with ATP could be useful for monitoring of metabolic processes.
Anton V Dubrovskiy et al.
The Journal of organic chemistry, 77(24), 11232-11256 (2012-12-05)
A novel, efficient route to biologically and pharmaceutically important o-(dimethylamino)aryl ketones, acridones, acridinium salts, and 1H-indazoles has been developed starting from readily available hydrazones of aldehydes and o-(trimethylsilyl)aryl triflates. The reaction proceeds through arynes under mild conditions, tolerates a wide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service