All Photos(2)
About This Item
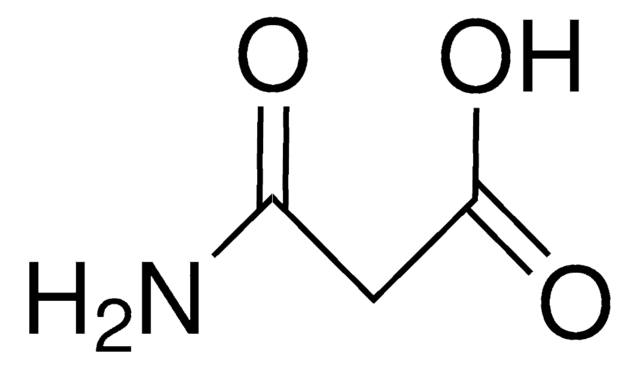
Linear Formula:
CH2(CONH2)2
CAS Number:
Molecular Weight:
102.09
Beilstein:
1751401
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
172-175 °C (lit.)
fluorescence
λex 367 nm; λem 445 nm (α-keto acid adduct)
SMILES string
NC(=O)CC(N)=O
InChI
1S/C3H6N2O2/c4-2(6)1-3(5)7/h1H2,(H2,4,6)(H2,5,7)
InChI key
WRIRWRKPLXCTFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The malonamide derivatives are obtained by the one-pot, five-component condensation reaction of isocyanide, Meldrum′s acid, arylidene malononitrile, and two amine molecules in CH2Cl2.
Application
The malonamide-based ionic liquid extractant was used in the extraction of europium(iii) and other trivalent rare-earth ions from nitric acid medium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M M Schiavoni et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 56A(8), 1533-1541 (2000-07-25)
The conformational and tautomeric compositions of malonamide, NH2-C(O)-CH2-C(O)-NH2 were determined by vibrational spectroscopy and theoretical calculations (HF/6-31G*, B3PW91/6-31G*). Solid state Fourier transform infrared and Raman spectra were analysed. They reveal the existence of a diketo tautomer. Theoretical calculations predict a
Ana G Neo et al.
Molecular diversity, 15(2), 529-539 (2010-09-03)
A general synthesis of 1,3-dicarbonylic compounds using multicomponent reactions of isocyanides is described. The process involves a Passerini three-component condensation of glyoxal derivatives, isocyanides and acetic acid, followed by metal mediated reductive or solvolytic removal of the acid component. Noteworthy
Syntheses and antiinflammatory activity of malonamic acid, malonamate and malonamide derivatives of some heterocyclic compounds.
T Katagi et al.
Chemical & pharmaceutical bulletin, 33(11), 4878-4888 (1985-11-01)
Mi-Hyun Kim et al.
Organic letters, 12(12), 2826-2829 (2010-05-27)
A new enantioselective synthetic method of (-)-paroxetine is reported. (-)-Paroxetine could be obtained in 15 steps (95% ee and 9.1% overall yield) from N,N-bis(p-methoxyphenyl)malonamide tert-butyl ester via the enantioselective phase-transfer catalytic alkylation and the diastereoselective Michael addition as the key
M J Barlow et al.
Solid state nuclear magnetic resonance, 1(4), 197-204 (1992-11-01)
Methyl tunnel frequencies, measured at 4 K, are found to be 455 +/- 8 kHz in methyl malonamide and 496 +/- 8 kHz in methyl ethyl ketone. The first is unaffected by deuteration of the amide groups. Measurements of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service