SML2332
Dynarrestin
≥98% (HPLC)
Synonym(s):
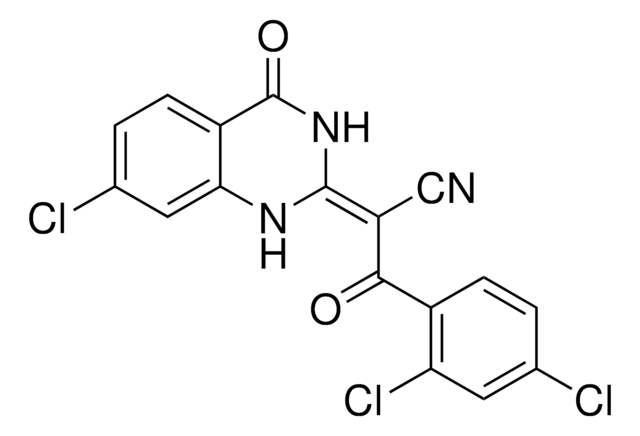
4-(4-Diethylaminophenyl)-2-(2,4-difluoro-phenylamino)-thiazole-5-carboxylic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H23F2N3O2S
CAS Number:
Molecular Weight:
431.50
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to brown
solubility
DMSO: 2 mg/mL, clear
storage temp.
2-8°C
SMILES string
CCN(CC)C1=CC=C(C2=C(C(OCC)=O)SC(NC3=C(F)C=C(F)C=C3)=N2)C=C1
Biochem/physiol Actions
Dynarrestin is a potent and selective reversible inhibitor of cytoplasmic dyneins 1 and 2 that inhibits dynein 1-dependent microtubule binding and motility without affecting ATP hydrolysis. It inhibits endosome movement and disturbs mitosis in cells. Dynarrestin inhibits dynein 2-mediated intraflagellar transport of the cargo IFT88 and flux of Smo within cilia. Also it inhibits cancer cells proliferation downstream of Smo.
Dynarrestin is an aminothiazole derivative, which can bind to protein tyrosine phosphatase interacting protein 51 (PTPIP51). This interaction helps in regulating various signaling pathways that lead to proliferation and migration. Dynarrestin blocks hedgehog (Hh)-dependent signaling in mouse and human cells.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Eric Dietel et al.
PloS one, 14(5), e0216642-e0216642 (2019-05-11)
LDC3/Dynarrestin, an aminothiazole derivative, is a recently developed small molecule, which binds protein tyrosine phosphatase interacting protein 51 (PTPIP51). PTPIP51 interacts with various proteins regulating different signaling pathways leading to proliferation and migration. Her2 positive breast cancer cells (SKBR3) express
Nicholas M Zehrbach et al.
Molecular biology of the cell, 34(7), ar65-ar65 (2023-04-13)
Rab GTPase-mediated vesicle trafficking of cell surface proteins, including integrins, through endocytic and recycling pathways is important in controlling cell-extracellular matrix interactions during cell migration. The focal adhesion adaptor protein, paxillin, plays a central role in regulating adhesion dynamics and
Susanne Höing et al.
Cell chemical biology, 25(4), 357-369 (2018-02-06)
Aberrant hedgehog (Hh) signaling contributes to the pathogenesis of multiple cancers. Available inhibitors target Smoothened (Smo), which can acquire mutations causing drug resistance. Thus, compounds that inhibit Hh signaling downstream of Smo are urgently needed. We identified dynarrestin, a novel
Ariane Schleinitz et al.
Cell reports, 42(1), 111969-111969 (2023-01-15)
The transfer of endocytosed cargoes to lysosomes (LYSs) requires HOPS, a multiprotein complex that tethers late endosomes (LEs) to LYSs before fusion. Many proteins interact with HOPS on LEs/LYSs. However, it is not clear whether these HOPS interactors localize to
Yunan Ye et al.
EMBO reports, 23(11), e55251-e55251 (2022-10-11)
Microtubules typically promote nuclear centring during early embryonic divisions in centrosome-containing vertebrates. In acentrosomal mouse zygotes, microtubules also centre male and female pronuclei prior to the first mitosis, this time in concert with actin. How nuclear centring is brought about
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service