17773
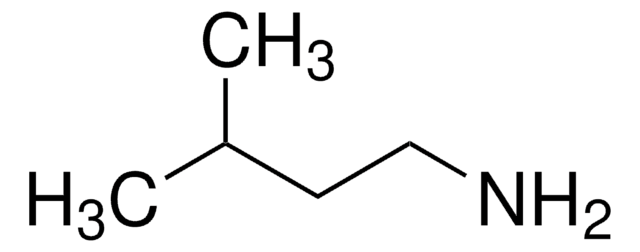
1-Amino-3-methylbutane hydrochloride
puriss., ≥98.0% (TLC)
Synonym(s):
Isopentylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH2CH2NH2 · HCl
CAS Number:
Molecular Weight:
123.62
Beilstein:
3947083
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
puriss.
Quality Level
Assay
≥98.0% (TLC)
form
solid
mp
65-69 °C
SMILES string
Cl.CC(C)CCN
InChI
1S/C5H13N.ClH/c1-5(2)3-4-6;/h5H,3-4,6H2,1-2H3;1H
InChI key
HOMVDRDAAUYWKL-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Tavakkol et al.
Journal of chromatography, 274, 37-44 (1983-05-13)
Columns of Chromosorb 103, Tenax-GC, Amine 220 plus potassium hydroxide on Chromosorb W, and Carbowax 20M plus potassium hydroxide on Chromosorb W were compared for their ability to separate bacterial amines as their free bases in aqueous solution. A 1.52
C Ji et al.
Carcinogenesis, 7(2), 301-303 (1986-02-01)
Nitrosomethylisoamylamine (NMIA), a carcinogenic N-nitroso compound was synthesized from isoamylamine (IAA) in a glucose-ammonium nitrate medium after several days' incubation with fungi and subsequent nitrosation with sodium nitrate. The nitrosamine was not produced by control reactions which lacked either IAA
[Studies on the synthesis of two new nitrosamines (NMAMBA and NMAMPA) from isoamylamine and sodium nitrite by fungi].
S J Lu et al.
Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae, 8(4), 255-260 (1986-08-01)
The deamination of isoamylamine by monamine oxidase in mitochondrial preparations from rat liver and heart: a comparison with phenylethylamine.
E M Peers et al.
Biochemical pharmacology, 29(8), 1097-1102 (1980-04-15)
Gavin L Sacks et al.
Analytical chemistry, 75(20), 5495-5503 (2004-01-09)
We report an automated method for high-precision position-specific isotope analysis (PSIA) of carbon in amino acid analogues. Carbon isotope ratios are measured for gas-phase pyrolysis fragments from multiple sources of 3-methylthiopropylamine (3MTP) and isoamylamine (IAA), the decarboxylated analogues of methionine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service