ABN454
Anti-Tau (T22), oligomeric Antibody
serum, from rabbit
Synonym(s):
Microtubule-associated protein tau oligomer, Tau oligomer, PHF-tau oligomer, Paired helical filament-tau oligomer, Neurofibrillary tangle protein oligomer
About This Item
Recommended Products
biological source
rabbit
Quality Level
antibody form
serum
antibody product type
primary antibodies
clone
polyclonal
species reactivity
human
technique(s)
ELISA: suitable
dot blot: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
neutralization: suitable
western blot: suitable
NCBI accession no.
UniProt accession no.
shipped in
wet ice
target post-translational modification
unmodified
Gene Information
human ... MAPT(4137)
Related Categories
General description
Specificity
Immunogen
Application
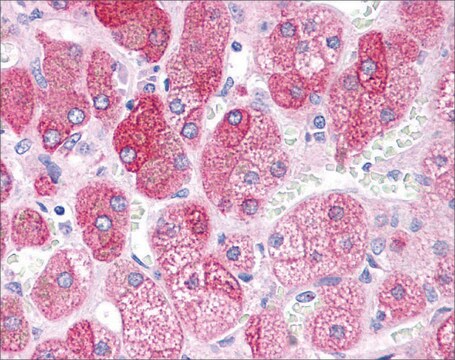
Immunohistochemistry Analysis: A 1:245-1,000 dilution from a representative lot detected tau oligomers in frontal cortices from Alzheimer′s Diseased (AD) and Lewy Body Diseased (LBD) patients (Courtesy of Prof. Rakez Kayed, University of Texas, Galveston).
Immunofluorescence Analysis: A representative lot detected oligomeric tau in chronic traumatic encephalopathy (CTE) brain tissue sections, while little or no tau oligomer immunoreactivity was seen in non-CTE human brain sections. The tau oligomer immunoreactivity colocalized with that of tau pThr231 with a cis conformation, but not tau pThr231 with a trans conformation, between pThr231 and Pro232 (Kondo, A., et al. (2015). Nature. 523(7561):431-436).
Immunofluorescence Analysis: A representative lot detected oligomeric tau immunoreactivity in paraffin-embedded frontal cortex sections from Alzheimer′s diseased (AD) brains (Lasagna-Reeves, C. A., et al. (2012). FASEB J. 26(5):1946-1959).
Western Blotting Analysis: A 1:1,000 dilution from a representative lot detected oligomeric tau in chronic traumatic encephalopathy (CTE) in Tau Aggregate lysate.
Western Blotting Analysis: Representative lots detected oligomeric tau, but not monomeric tau, or any other oligomeric and fibrillar proteins (Wu J.W., et al. (2013). J. Biol. Chem. 288(3):1856-1870; Lasagna-Reeves, C. A., et al. (2012). FASEB J. 26(5):1946-1959).
Dot Blot Analysis: A representative lot detected tau oligomers, but not tau monomer or paired helical filaments (PHFs) (Lasagna-Reeves, C. A., et al. (2012). FASEB J. 26(5):1946-1959).
ELISA Analysis: Representative lots detected vitro formed tau oligomers as well as tau oligomers in PBS-soluble brain extracts from progressive supranuclear palsy (PSP) patients (Lasagna-Reeves, C.A., et al. (2012). Sci. Rep. 2:700; Lasagna-Reeves, C. A., et al. (2012). FASEB J. 26(5):1946-1959).
ELISA Analysis: A representative lot showed selective reactivity toward oligomeric tau, while exhibiting greatly reduced immunoreactivity toward tau fibrils, and no reactivity toward monomeric tau, or other protein oligomers or fibrils (e.g., Aβ, α-synuclein, or islet amyloid polypeptide) (Lasagna-Reeves, C. A., et al. (2012). FASEB J. 26(5):1946-1959).
Immunoprecipitation Analysis: Representative lots immunoprecipitated oligomeric tau from Alzheimer′s Diseased (AD), but not non-AD brains (Lasagna-Reeves, C.A., et al. (2012). Sci. Rep. 2:700; Lasagna-Reeves, C. A., et al. (2012). FASEB J. 26(5):1946-1959).
Neutralizing Analysis: Representative lots neutralized oligomeric tau toxicity to SH-SY5Y human neuroblastoma cells (Lasagna-Reeves, C.A., et al. (2012). Sci. Rep. 2:700; Lasagna-Reeves, C. A., et al. (2012). FASEB J. 26(5):1946-1959).
Immunohistochemistry Analysis: A representative lot detected oligomeric tau immunoreactivity in paraffin-embedded brain sections from progressive supranuclear palsy (PSP) patients (Lasagna-Reeves, C. A., et al. (2012). FASEB J. 26(5):1946-1959).
Neuroscience
Neurodegenerative Diseases
Quality
Western Blotting Analysis: A 1:1,000 dilution of this antibody detected tau oligomers in Alzheimer′s diseased (AD), but not non-AD, human brain tissue lysate.
Target description
Physical form
Storage and Stability
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze/thaw cycles, which may damage IgG and affect product performance.
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![3-[1,3-Dihydro-4-(5-hydroxy-1-pentyn-1-yl)-1-oxo-2H-isoindol-2-yl]-2,6-piperidinedione ≥95.0%](/deepweb/assets/sigmaaldrich/product/structures/165/184/ebc29f1b-f63f-4e48-afb5-b3aa4c69795a/640/ebc29f1b-f63f-4e48-afb5-b3aa4c69795a.png)