D-049
N-Desmethylclobazam solution
100 μg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
100 μg/mL in acetonitrile
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
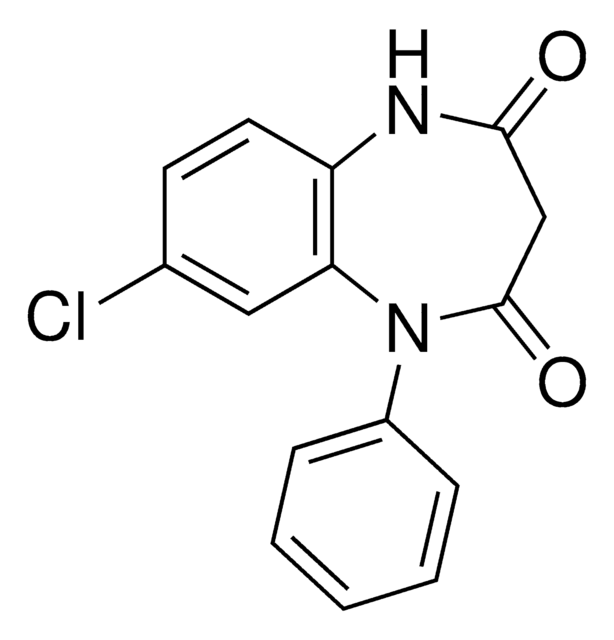
SMILES string
O=C(C1)N(C2=CC=CC=C2)C3=C(C=CC(Cl)=C3)NC1=O
InChI
1S/C15H11ClN2O2/c16-10-6-7-12-13(8-10)18(11-4-2-1-3-5-11)15(20)9-14(19)17-12/h1-8H,9H2,(H,17,19)
InChI key
RRTVVRIFVKKTJK-UHFFFAOYSA-N
General description
Application
- Impact on bioavailability in epilepsy treatment: A study discussed the implications of N-Desmethylclobazam on drug to drug interactions and neuropsychological impact when used as add-on therapy in drug-resistant epilepsies, highlighting its role in enhancing therapeutic bioavailability (Pietrafusa et al., 2023).
- Research on pharmacokinetic variability: Research explored the pharmacokinetic variability of N-Desmethylclobazam, emphasizing its influence by age, drug interactions, and biochemical markers of toxicity in patients with epilepsy, contributing to a deeper understanding of its pharmacological properties (Heger et al., 2024).
- Evaluation of drug-drug interactions: A comprehensive analysis investigated potential drug-drug interactions between N-Desmethylclobazam and anti-seizure medications, providing insights into its safe and effective use in clinical settings (Gaston et al., 2023).
- Synergistic effects in epilepsy management: A multicenter study on the cenobamate-clobazam interaction revealed both pharmacokinetic interactions and evidence of synergy, underscoring N-Desmethylclobazam′s potential to enhance treatment outcomes in epilepsy (Osborn and Abou-Khalil, 2023).
- Analysis of metabolites in forensic science: Research on the measurement of benzodiazepine receptor agonists′ metabolites, including N-Desmethylclobazam, outlined its detection and concentration levels in urine, offering applications in forensic toxicology (Volkova et al., 2023).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
35.6 °F - closed cup
Flash Point(C)
2 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service