89744
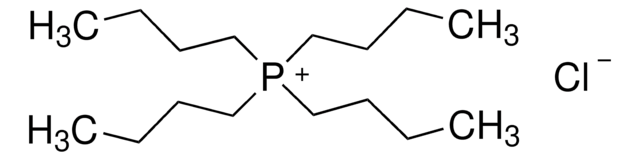
Trihexyltetradecylphosphonium chloride
≥95.0% (NMR)
Synonym(s):
Tetradecyltrihexylphosphonium chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[CH3(CH2)5]3P(Cl)(CH2)13CH3
CAS Number:
Molecular Weight:
519.31
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0% (NMR)
form
liquid
density
0.895 g/mL at 20 °C (lit.)
functional group
phosphine
SMILES string
[Cl-].CCCCCCCCCCCCCC[P+](CCCCCC)(CCCCCC)CCCCCC
InChI
1S/C32H68P.ClH/c1-5-9-13-17-18-19-20-21-22-23-24-28-32-33(29-25-14-10-6-2,30-26-15-11-7-3)31-27-16-12-8-4;/h5-32H2,1-4H3;1H/q+1;/p-1
InChI key
JCQGIZYNVAZYOH-UHFFFAOYSA-M
Related Categories
General description
Trihexyltetradecylphosphonium chloride is a hydrophobic ionic liquid, which is soluble in carbon dioxide.
Application
Trihexyltetradecylphosphonium chloride can be used as binder to develop a silver particle-modified carbon paste electrode, which can determine nitrite ions at low concentration.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
244.4 °F
Flash Point(C)
118 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Surprisingly high solubility of the ionic liquid trihexyltetradecylphosphonium chloride in dense carbon dioxide.
Hutchings JW, et al.
Green Chemistry, 7(6), 475-478 (2005)
Pan Sun et al.
Journal of colloid and interface science, 539, 214-222 (2018-12-24)
Separation and recycling of rare-earths using ionic liquids as extractant are becoming a promising approach to replace traditional volatile organic solvents in recent years. Generally, the addition of some special salts could improve the extraction efficiency of rare-earths by numerous
Supercritical CO 2 as an efficient medium for layered silicate organomodification: preparation of thermally stable organoclays and dispersion in polyamide 6
Naveau, Elodie, et al.
Polymer, 50.6, 1438-1446 (2009)
Maria Mazzucotelli et al.
Journal of chromatography. A, 1583, 124-135 (2018-11-28)
Room-temperature ionic liquids (ILs) have been shown to be successful as stationary phases (SPs) for gas chromatography in several fields of applications because of their unique and tunable selectivity, low vapor pressure and volatility, high thermal stability (over 300 °C), and
Paula Berton et al.
Molecules (Basel, Switzerland), 26(1) (2020-12-31)
In this study, three magnetic ionic liquids (MILs) were investigated for extraction of four estrogens, i.e., estrone (E1), estradiol (E2), estriol (E3), and ethinylestradiol (EE2), from environmental water. The cation trihexyl(tetradecyl)phosphonium ([P66614]+), selected to confer hydrophobicity to the resulting MIL
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service