76172
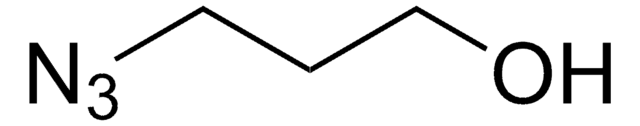
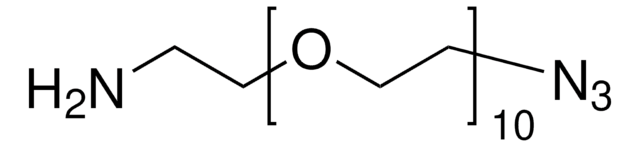
O-(2-Aminoethyl)-O′-(2-azidoethyl)pentaethylene glycol
≥90% (oligomer purity)
Synonym(s):
Azido-PEG-amine (n=6)
About This Item
Recommended Products
Quality Level
Assay
≥90% (oligomer purity)
form
liquid
reaction suitability
reaction type: click chemistry
reagent type: cross-linking reagent
functional group
amine
azide
storage temp.
2-8°C
SMILES string
NCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-]
InChI
1S/C14H30N4O6/c15-1-3-19-5-7-21-9-11-23-13-14-24-12-10-22-8-6-20-4-2-17-18-16/h1-15H2
InChI key
VCQSTKKJKNUQBI-UHFFFAOYSA-N
Application
- As a reactant for the synthesis of thin films of azide functionalized poly(L-glutamic acid) for drug delivery.
- For the synthesis of curcumin monoazide derivatives for fabricating biologically active curcumin conjugates.
- To modify the surface of TiO2 nanoparticles to attach DNA strands for photocatalytic reduction of CO2.
- For the conjugation of poly(ethylene glycol) (PEG) with toll-like receptor 7 (TLR7) to improve its pharmaceutical properties.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)