All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H7NO3
CAS Number:
Molecular Weight:
201.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
300-304 °C (lit.)
functional group
ester
nitrile
SMILES string
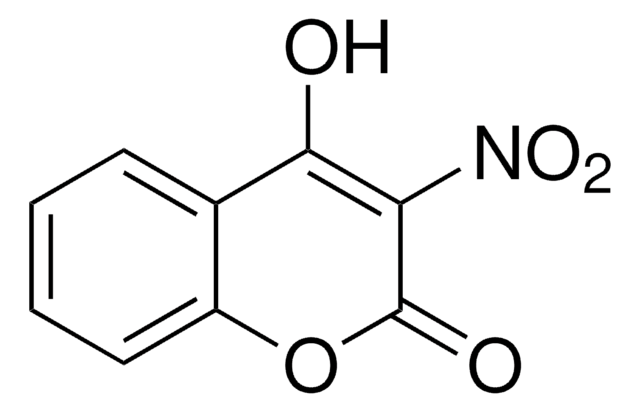
CC1=C(C#N)C(=O)Oc2cc(O)ccc12
InChI
1S/C11H7NO3/c1-6-8-3-2-7(13)4-10(8)15-11(14)9(6)5-12/h2-4,13H,1H3
InChI key
GLGBPOSQDAZSIZ-UHFFFAOYSA-N
General description
3-Cyano-7-hydroxy-4-methylcoumarin has been isolated from Curcuma longa isopropanol extract.
Application
3-Cyano-7-hydroxy-4-methylcoumarin (CHMC) may be used as reagent for the stereo-specific preparation of RP- and SP-O-alkyl methylphosphonyl-CHMC esters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sudha Ponnusamy et al.
Evidence-based complementary and alternative medicine : eCAM, 2011, doi:10-doi:10 (2010-10-19)
Pancreatic α-amylase inhibitors offer an effective strategy to lower the levels of post prandial hyperglycemia via control of starch breakdown. Eleven Ayurvedic Indian medicinal plants with known hypoglycemic properties were subjected to sequential solvent extraction and tested for α-amylase inhibition
Y Ashani et al.
Chemico-biological interactions, 187(1-3), 362-369 (2010-03-23)
Fluorogenic organophosphate inhibitors of acetylcholinesterase (AChE) homologous in structure to nerve agents provide useful probes for high throughput screening of mammalian paraoxonase (PON1) libraries generated by directed evolution of an engineered PON1 variant with wild-type like specificity (rePON1). Wt PON1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service