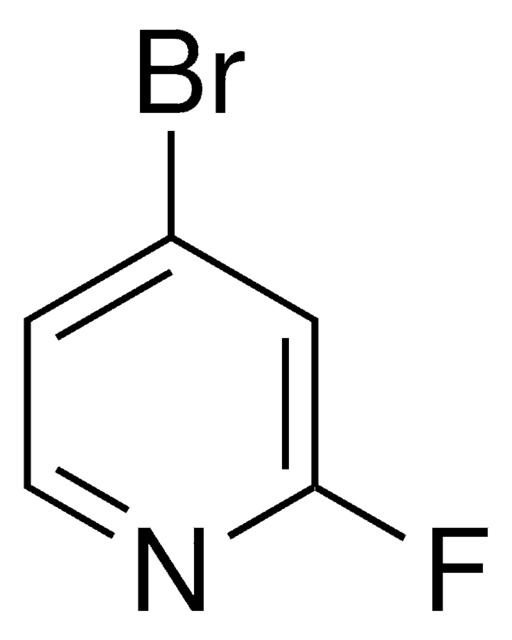
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H6N2
CAS Number:
Molecular Weight:
118.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.626 (lit.)
bp
103 °C/1 mmHg (lit.)
density
1.165 g/mL at 25 °C (lit.)
SMILES string
c1ccn2ccnc2c1
InChI
1S/C7H6N2/c1-2-5-9-6-4-8-7(9)3-1/h1-6H
InChI key
UTCSSFWDNNEEBH-UHFFFAOYSA-N
Related Categories
General description
In vivo anti-trypanosomal activity of imidazo[1,2-a]pyridiness in the STIB900 mouse model has been investigated.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enza Palazzo et al.
Neuropharmacology, 58(3), 660-667 (2009-12-01)
The 6-methoxy-2-phenylimidazo[1,2-b]pyridazine-3-carboxylic acid, DM2, exerts anti-absence activity and blocks Cav3.1 channel, a T-type voltage-dependent Ca(2+) channel subtype, in vitro. The current study investigated the effect of intra-ventrolateral periaqueductal grey (VLPAG) administration of DM2 on formalin-induced nocifensive responses in rats. In
Yoshito Terao et al.
Bioorganic & medicinal chemistry letters, 22(24), 7326-7329 (2012-11-14)
Imidazo[1,2-a]pyridine derivatives were designed, synthesized, and evaluated as inhibitors of the apoptosis signal-regulating kinase 1 (ASK1). These were based on a benzothiazole derivative that was discovered from high-throughput screening of our compound library. As a result, we identified potent, selective
Nick Bailey et al.
Bioorganic & medicinal chemistry letters, 19(13), 3602-3606 (2009-05-27)
Acid pump antagonists (APAs) such as the imidazo[1,2-a]pyridine AZD-0865 2 have proven efficacious at low oral doses in acid related gastric disorders. Herein we describe some of the broader SAR in this class of molecule and detail the discovery of
Mohamed A Ismail et al.
Bioorganic & medicinal chemistry, 16(2), 683-691 (2007-11-03)
The key dinitrile intermediates 4a-d were synthesized by reaction of phenacyl bromide 1 and the appropriate 2-amino-5-bromopyridines to yield 3a-d. Suzuki coupling of 3a-d with 4-cyanophenylboronic acid yielded the 2,6-bis(4-cyanophenyl)-imidazo[1,2-a]pyridine derivatives 4a-d. The bis-amidoximes 5a-d, obtained from 4a-d by the
Suren Husinec et al.
Organic letters, 13(9), 2286-2289 (2011-03-31)
A base promoted cyclization of the protected N-propargylaminopyridines was shown to be an efficient method for the preparation of imidazo[1,2-a]pyridine derivatives. The reactions were carried out with a small excess of base, at room temperature or slightly above producing the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-phenylimidazo[1,2-a]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/281/247/6c2550a0-2f0c-4866-83d8-3c1fb039e165/640/6c2550a0-2f0c-4866-83d8-3c1fb039e165.png)
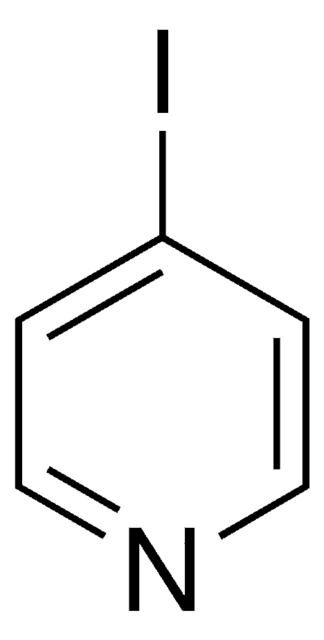
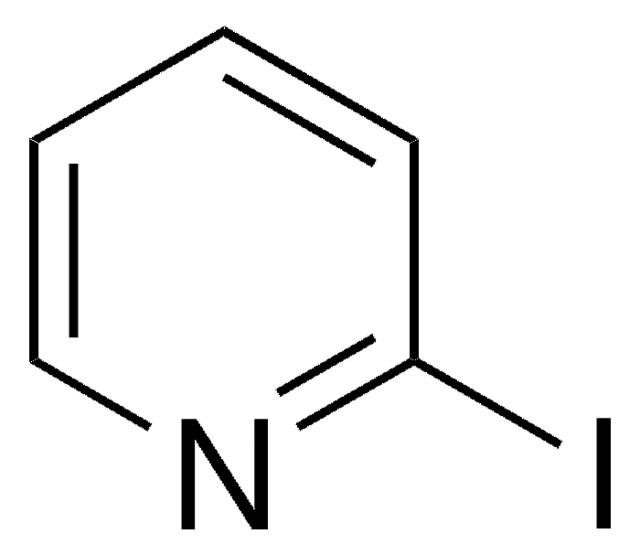
![Imidazo[1,2-a]pyrazine 97%](/deepweb/assets/sigmaaldrich/product/structures/370/804/1712d71f-52fb-4758-9a22-85b6c96cd4e8/640/1712d71f-52fb-4758-9a22-85b6c96cd4e8.png)
![Imidazo[1,2-a]pyrimidine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/187/001/4862c14e-bec7-4475-85a5-f178e48ff60f/640/4862c14e-bec7-4475-85a5-f178e48ff60f.png)