All Photos(1)
About This Item
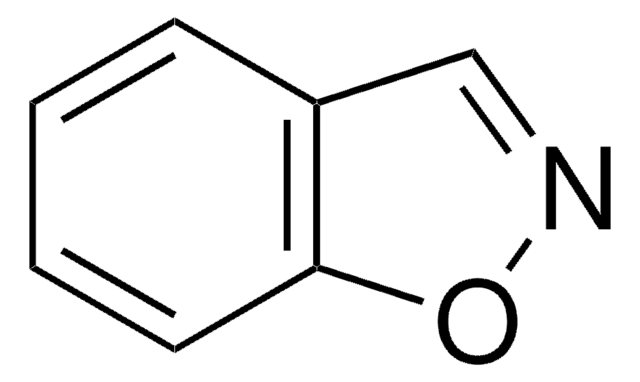
Empirical Formula (Hill Notation):
C7H5NO
CAS Number:
Molecular Weight:
119.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
refractive index
n20/D 1.584 (lit.)
bp
101-102 °C/15 mmHg (lit.)
density
1.183 g/mL at 25 °C (lit.)
SMILES string
c1ccc2nocc2c1
InChI
1S/C7H5NO/c1-2-4-7-6(3-1)5-9-8-7/h1-5H
InChI key
FZKCAHQKNJXICB-UHFFFAOYSA-N
General description
Anthranil undergoes thermal decomposition during single pulse shock-tube experiments to form aniline and cyclopentadiene carbonitrile. Surface-enhanced Raman spectrum of anthranil in activated silver colloid has been studied.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E V Kudryashova et al.
Biochimica et biophysica acta, 1550(2), 129-143 (2002-01-05)
Structure and dynamic properties of free poly(methacrylic acid) (PMA) and PMA complexed with alpha-chymotrypsin (CT) were studied using the time resolved fluorescence anisotropy technique. We have found that the interaction of PMA with CT induces the formation of a quasi-regular
A possible in vivo skin model for tumour promoter assays.
V Armuth et al.
Cancer letters, 15(3), 343-346 (1982-03-01)
Marna Pippel et al.
Bioorganic & medicinal chemistry letters, 19(22), 6376-6378 (2009-10-10)
In the previous article we demonstrated how certain CCK2R-selective anthranilic amides could be structurally modified to afford high-affinity, selective CCK1R activity. We now describe our efforts at modulating and optimizing the CCK1R and CCK2R affinities aimed at producing compounds with
Further exploration of stages in carcinogenesis.
V Armuth et al.
Carcinogenesis; a comprehensive survey, 7, 41-42 (1982-01-01)
Marna Pippel et al.
Bioorganic & medicinal chemistry letters, 19(22), 6373-6375 (2009-10-09)
A series of CCK2R-selective anthranilic amides is shown to derive CCK1R affinity via selective substitution of the amide side chain. Thus, extending the length of the original benzamide side chain by a single methylene unit imparts CCK1R affinity to the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service