All Photos(1)
About This Item
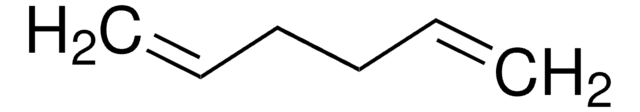
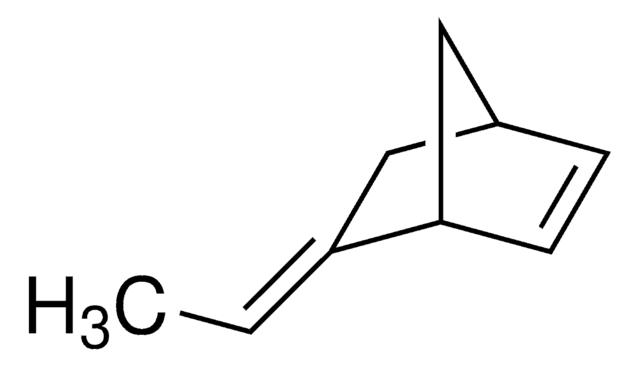
Linear Formula:
CH2=CH(CH2)4CH=CH2
CAS Number:
Molecular Weight:
110.20
Beilstein:
605288
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.422 (lit.)
bp
114-121 °C (lit.)
density
0.746 g/mL at 25 °C (lit.)
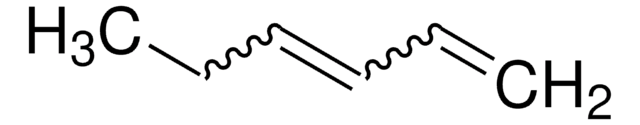
SMILES string
C=CCCCCC=C
InChI
1S/C8H14/c1-3-5-7-8-6-4-2/h3-4H,1-2,5-8H2
InChI key
XWJBRBSPAODJER-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,7-Octadiene can serve as a crosslinker and source of ethylene for a variant of Mori′s conditions in CEYM-related reactions.
Application
1,7-Octadiene has been used in a study to assess the structure and reaction rate in olefin ring-closing metathesis of a series of simple dienes. It has also been used in a study to investigate micropatterned surfaces prepared by plasma polymerization.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
50.0 °F - closed cup
Flash Point(C)
10 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Filová et al.
Physiological research, 58(5), 669-684 (2008-12-20)
Micropatterned surfaces have been used as a tool for controlling the extent and strength of cell adhesion, the direction of cell growth and the spatial distribution of cells. In this study, chemically micropatterned surfaces were prepared by successive plasma polymerization
Santos Fustero et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(35), 10991-10997 (2012-08-02)
The use of 1,7-octadiene as an in situ source of ethylene led us to develop a novel multicomponent tandem cross-enyne metathesis (CEYM)-Diels-Alder reaction. The process can be considered a relay metathesis, in which the ethylene liberated in the ring-closing metathesis
Ian W Ashworth et al.
Chemical communications (Cambridge, England), 46(38), 7145-7147 (2010-09-08)
In the RCM reactions of a series of simple α,ω-dienes, the relative order of reactivity has been unambiguously determined showing that cyclohexene forms faster than cyclopentene or cycloheptene. 1,5-Hexadiene inhibits the RCM of 1,7-octadiene; 1,5-hexadiene cannot progress to the RCM
Christopher Jay T Robidillo et al.
Nanoscale, 10(39), 18706-18719 (2018-10-03)
This study reports the preparation of functional bioinorganic hybrids, through application of the thiol-ene reaction, that exhibit catalytic activity and photoluminescent properties from enzymes and freestanding silicon nanocrystals. Thermal hydrosilylation of 1,7-octadiene and alkene-terminated poly(ethylene oxide)methyl ether with hydride-terminated silicon
M Ramiasa-MacGregor et al.
Nanoscale, 8(8), 4635-4642 (2016-02-09)
The wetting of a material can be tuned by changing the roughness on its surface. Recent advances in the field of nanotechnology open exciting opportunities to control macroscopic wetting behaviour. Yet, the benchmark theories used to describe the wettability of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service