D5392
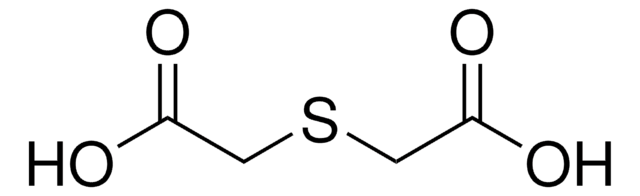
Dithiodiglycolic acid
≥98%
Synonym(s):
2,2′-Dithiodiacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6O4S2
CAS Number:
Molecular Weight:
182.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
form
powder
storage temp.
−20°C
SMILES string
OC(=O)CSSCC(O)=O
InChI
1S/C4H6O4S2/c5-3(6)1-9-10-2-4(7)8/h1-2H2,(H,5,6)(H,7,8)
InChI key
DLLMHEDYJQACRM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Enhancement of octacalcium phosphate via Dithiodiglycolic acid: Research on the interlayer expansion of octacalcium phosphate illustrates the forced oxidation of Dithiodiglycolic acid intercalated molecules within its interlayers, showcasing a novel application in material science and chemical engineering (Sugiura et al., 2023).
- Drug delivery systems: A study on redox-responsive self-assembly PEG nanoparticles enhanced by triptolide, where Dithiodiglycolic acid plays a critical role, demonstrates its potential in improving the efficacy of antitumor treatments, providing insights into pharmaceutical applications (Wang et al., 2018).
- Synthesis and medicinal applications: A comprehensive study on the synthesis and biological evaluation of Dithiodiglycolic acid derivatives via oxidative coupling of thiols highlights its utility in medicinal chemistry, offering new avenues for drug design and development (Bakulina et al., 2019).
- Redox-sensitive coordination polymers: The development of redox-sensitive nanoscale coordination polymers for drug delivery and cancer theranostics utilizes Dithiodiglycolic acid, providing significant advancements in cancer treatment strategies (Zhao et al., 2017).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Huiyun Zhang et al.
International journal of pharmaceutics, 575, 118980-118980 (2020-01-04)
Cardiac glycosides (CGs) have been used to treat cancer for hundreds of years. However, the narrow therapeutic window and system toxicity have hindered their wide clinical applications. Herein, the small molecule prodrug strategy and nanotechnology were integrated into one drug
Shenwu Zhang et al.
Drug delivery, 24(1), 1460-1469 (2017-09-28)
Breast cancer leads to high mortality of women in the world. Docetaxel (DTX) has been widely applied as one of the first-line chemotherapeutic drugs for breast cancer therapy. However, the clinical outcome of DTX is far from satisfaction due to
Huiyun Zhang et al.
Drug development and industrial pharmacy, 46(11), 1800-1808 (2020-09-25)
Curcumin (CUR), a nontoxic natural compound with potent antitumor activity, was limited in clinical application due to its insolubility and exceedingly low bioavailability. In this study, a novel prodrug-nanoparticle (CSSV/TPGS-NPs) self-assembled by co-nanoprecipitation of CUR-s-s-vitamin E conjugate and d-alpha-tocopheryl polyethylene
Huiyun Zhang et al.
Drug delivery, 24(1), 1170-1178 (2017-08-25)
Periplocymarin (PPM), a cardiac glycoside, has a narrow therapeutic index, poor tumor selectivity and severe cardiovascular toxicity which hinder its wide clinical applications in cancer treatment. Herein, we report novel redox-responsive prodrug-nanoparticles (MPSSV-NPs) self-assembled by co-nanoprecipitation of PPM-vitamin E conjugate
Qingqing Xiong et al.
Frontiers in pharmacology, 9, 61-61 (2018-03-01)
Combination of doxorubicin with sorafenib (SF) was reported to be a promising strategy for treating hepatocellular carcinoma (HCC). In this study, we designed a reduction-responsive supramolecular nanosystem based on poly (ethylene glycol)-β-cyclodextrin (PEG-CD) and a disulfide-containing adamantine-terminated doxorubicin prodrug (AD)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service