All Photos(1)
About This Item
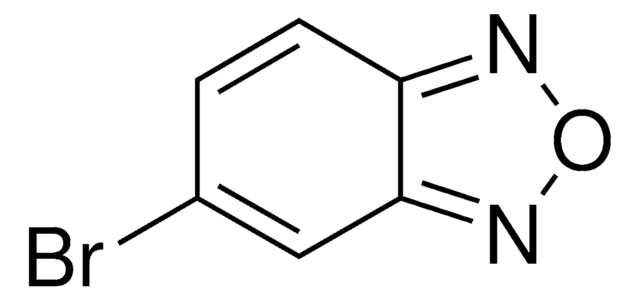
Empirical Formula (Hill Notation):
C6H4N2O
CAS Number:
Molecular Weight:
120.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
75-85 °C/20 mmHg (lit.)
mp
47-51 °C (lit.)
SMILES string
c1ccc2nonc2c1
InChI
1S/C6H4N2O/c1-2-4-6-5(3-1)7-9-8-6/h1-4H
InChI key
AWBOSXFRPFZLOP-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
168.8 °F - closed cup
Flash Point(C)
76 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Santa et al.
Biomedical chromatography : BMC, 12(2), 73-77 (1998-05-06)
The enantiomneric separation and the detection of 2-arylpropionic acids after derivatization with the fluorescent reagents with a benzofurazan structure, (S)-(+)-4-(N,N- dimethylaminosulphonyl)-7-(3-aminopyrrolidin-1-yl)-2,1,3-ben zoxadiazole ((S)-DBD-Apy), (R)-(-)-4-nitro-7-(3-aminopyrrolidin-1-yl)-2,1,3- benzoxadiazole ((R)-NBD-Apy), 4-N,N-dimethylaminosulphonyl-7-piperazino-2,1,3-benzoxadi zole (DBD-PZ) and N-hydrazinoformylmethyl-N-methylamino-4,4- N,N-dimethylaminosulphonyl-2,1,3-benzoxadiazole (DBD-CO-Hz) by high-performance liquid chromatography (HPLC) and electrospray
Fluorogenic reagents, having benzofurazan structure, in liquid chromatography.
K Imai et al.
Journal of pharmaceutical and biomedical analysis, 7(12), 1395-1403 (1989-01-01)
S Uchiyama et al.
Biomedical chromatography : BMC, 15(5), 295-318 (2001-08-17)
Fluorogenic and fluorescent labeling reagents having a benzofurazan (2,1,3-benzoxadiazole) skeleton such as 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F), 4-N,N-dimethylaminosulfonyl-7-fluoro-2,1,3-benzoxadiazole (DBD-F), 4-aminosulfonyl-7-fluoro-2,1,3-benzoxadiazole (ABD-F), ammonium 7-fluoro-2,1,3-benzoxadiazole-4-sulfonate (SBD-F), 4-hydrazino-7-nitro-2,1,3-benzoxadiazole (NBD-H), 4-N,N-dimethylaminosulfonyl-7-hydrazino-2,1,3-benzoxadiazole (DBD-H), 4-nitro-7-N-piperazino-2,1,3-benzoxadiazole (NBD-PZ), 4-N,N-dimethylaminosulfonyl-7-N-piperazino-2,1,3-benzoxadiazole (DBD-PZ), 4-(N-chloroformylmethyl-N-methyl)amino-7-N,N-dimethylaminosulfonyl-2,1,3-benzoxadiazole (DBD-COCl) and 7-N,N-dimethylaminosulfonyl-4-(2,1,3-benzoxadiazolyl) isothiocyanate (DBD-NCS) are reviewed in terms of
Tomofumi Santa et al.
Biomedical chromatography : BMC, 22(4), 343-353 (2007-12-07)
Chemical derivatization is often used to enhance the detectability of the target compounds and to improve the separation efficiency in high-performance liquid chromatography (HPLC). In this review, we describe the recent progress in the development of derivatization reagents having a
Alex Brown et al.
The journal of physical chemistry. A, 116(1), 46-54 (2011-12-06)
General chemical strategies which provide controlled changes in the emission or absorption properties of biologically compatible fluorophores remain elusive. One strategy employed is the conversion of a fluorophore-attached alkyne (or azide) to a triazole through a copper-catalyzed azide-alkyne coupling (CuAAC)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Benzo[c][1,2,5]oxadiazole-5-boronic acid pinacol ester 97%](/deepweb/assets/sigmaaldrich/product/structures/143/941/5b091bed-2dcd-4ac5-b86d-70df392aabce/640/5b091bed-2dcd-4ac5-b86d-70df392aabce.png)