All Photos(2)
About This Item
Empirical Formula (Hill Notation):
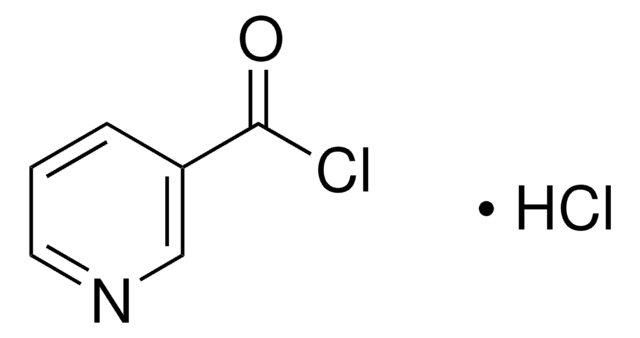
C6H3Cl2NO
CAS Number:
Molecular Weight:
176.00
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
48-51 °C (lit.)
SMILES string
ClC(=O)c1ccc(Cl)nc1
InChI
1S/C6H3Cl2NO/c7-5-2-1-4(3-9-5)6(8)10/h1-3H
InChI key
FMEBIWNKYZUWFV-UHFFFAOYSA-N
General description
6-Chloronicotinoyl chloride undergoes esterification reaction with diethylene glycol and pentaethylene glycol.
Application
6-Chloronicotinoyl chloride may be used to synthesize:
- [3H]imidacloprid (a candidate radioligand)
- [3H]acetamiprid
- N,N′-(1,4-phenylene)bis(6-(4-aminophenoxy) nicotinamide)
- 3-acetyl-6-chloropyridine
- 1,3-dipropyl-8(6-chloro-3-pyridyl)xanthine
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shinzo Kagabu et al.
Bioscience, biotechnology, and biochemistry, 67(5), 980-988 (2003-07-02)
The asymmetric chloronicotinyl insecticide, 1-[1-(6-chloro-3-pyridyl)ethyl]-2-nitroiminoimidazolidine, was prepared, and the absolute configurations of the enantiomers were determined by an X-ray analysis. The insecticidal activity against the housefly measured with metabolic inhibitors showed the (S) enantiomer to be slightly more active than
Nicotinic acid crown ethers. Synthesis and structural characterization of polyethereal macrocyclic lactones from 6-chloronicotinic acid.
Newkome GR, et al.
The Journal of Organic Chemistry, 45(26), 5423-5425 (1980)
Preparation of thermally stable, low dielectric constant, pyridine-based polyimide and related nanofoams.
Aram E and Mehdipour-Ataei S.
Journal of Applied Polymer Science, 128(6), 4387-4394 (2013)
[6-chloro-3-pyridylmethyl-3H] neonicotinoids as high-affinity radioligands for the nicotinic acetylcholine receptor: Preparation using NaB3H4 and LiB3H4.
Latli B, et al.
Journal of Labelled Compounds & Radiopharmaceuticals, 38(11)971-978, 971-978 (1996)
P J Scammells et al.
Journal of medicinal chemistry, 37(17), 2704-2712 (1994-08-19)
This report describes the synthesis of 29 xanthines containing a chemoreactive chloroaryl, beta-chloroethylamino, alpha,beta-unsaturated carbonyl, bromoacetyl, 3-(fluorosulfonyl)benzoyl, or 4-(fluorosulfonyl)benzoyl group as part of an exocyclic 1-, 3-, or 8-substituent. The xanthines inhibited the binding of [3H]-8-cyclopentyl-1,3-dipropylxanthine ([3H]CPX) to the A1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service