All Photos(1)
About This Item
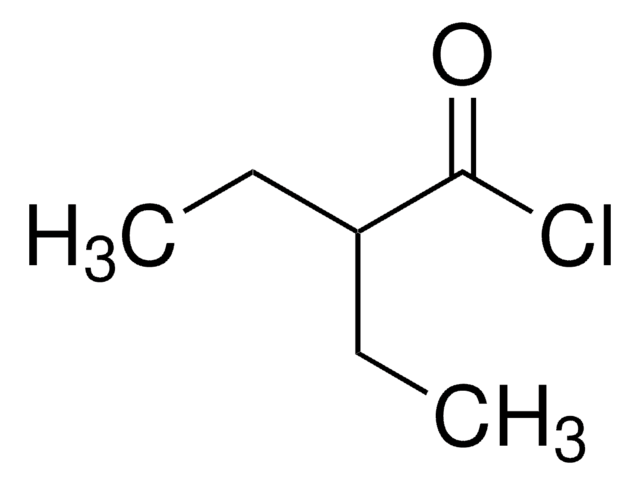
Linear Formula:
C2H5CH(CH3)COCl
CAS Number:
Molecular Weight:
120.58
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.418 (lit.)
bp
117-121 °C (lit.)
density
0.972 g/mL at 25 °C (lit.)
SMILES string
CCC(C)C(Cl)=O
InChI
1S/C5H9ClO/c1-3-4(2)5(6)7/h4H,3H2,1-2H3
InChI key
XRPVXVRWIDOORM-UHFFFAOYSA-N
Related Categories
General description
2-Methylbutyryl chloride is an acid chloride. It has been reported to be synthesized from 2-methylbutyric acid and thionyl chloride.
Application
2-Methylbutyryl chloride was used in the synthesis of 2-methylbutyramide and N-((R,S)-2-methylbutanoyl)salicylhydrazide.
It may be used in the synthesis of the 2-methyl-1-phenyl-1-butanone and 1,1′-(1,3,7,9-tetrahydroxydibenzofuran-2,6-diyl)bis(2-methylbutan-1-one).
It may be used in the synthesis of the 2-methyl-1-phenyl-1-butanone and 1,1′-(1,3,7,9-tetrahydroxydibenzofuran-2,6-diyl)bis(2-methylbutan-1-one).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
68.0 °F - closed cup
Flash Point(C)
20 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Modulation of the ring size and nuclearity of metallamacrocycles via the steric effect of ligands: preparation and characterization of 18-membered hexanuclear, 24-membered octanuclear, and 30-membered decanuclear manganese metalladiazamacrocycles with a-and ?-Branched N-Acylsalicylhydrazides.
John RP, et al.
Inorganic Chemistry, 44(20), 7109-7121 (2005)
Effects of sulphur nutrition during potato cultivation on the formation of acrylamide and aroma compounds during cooking.
Elmore JS, et al.
Food Chemistry, 122(3), 753-760 (2010)
Sabrina Heng et al.
European journal of medicinal chemistry, 45(4), 1478-1484 (2010-02-02)
Natural products often contain unusual scaffold structures that may be elaborated by combinatorial methods to develop new drug-like molecules. Visual inspection of more than 128 natural products with some type of anti-diabetic activity suggested that a subset might provide novel
Synthesis of 2-Methyl-2-hydroxy-1-phenyl-1-butanone.
Hu YX, et al.
Chemical World, 5, 10-10 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service