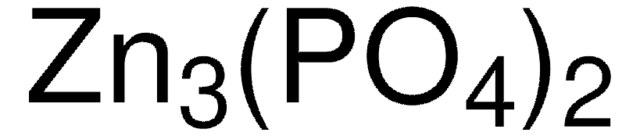04502
Zinc phosphide
≥19% active phoshor (P) basis, powder
Synonym(s):
Trizinc diphosphide, Zn3P2
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Zn3P2
CAS Number:
Molecular Weight:
258.12
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
form
powder
composition
active phoshor (P), ≥19%
reaction suitability
core: zinc
reagent type: catalyst
storage temp.
room temp
SMILES string
[Zn]=P[Zn]P=[Zn]
InChI
1S/2P.3Zn
InChI key
NQDYSWQRWWTVJU-UHFFFAOYSA-N
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Acute pulmonary edema and cardiac failure due to zinc phosphide ingestion.
Ozgur Sogut et al.
The Journal of emergency medicine, 40(6), e117-e118 (2009-08-18)
Rusen Yang et al.
Nano letters, 7(2), 269-275 (2007-02-15)
Hierarchical tree-shaped nanostructures, nanobelts, and nanowires of Zn3P2 were synthesized in a thermal assisted laser ablation process. All nanostructures are tetragonal phased Zn3P2 with excellent crystallinity and are free from an oxidization layer according to electron microscopy and X-ray diffraction
Jens Jacob et al.
Pest management science, 64(1), 74-80 (2008-01-15)
Zinc phosphide baits are used for controlling pest rodents but are also highly toxic to other vertebrates. The base for rodent baits containing zinc phosphide is usually wheat kernels which are highly attractive to birds. In this study, wheat-based pellets
I K El Zawawi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 94, 378-383 (2012-05-19)
The phase transformation of zinc phosphide (Zn(3)P(2)) thin films was detected through isochronal annealing process. The effects on isochronal annealing on the internal structural, optical and electrical properties of deposited Zn(3)P(2) thin films have been discussed. The films were prepared
C A Bhadkambekar et al.
Journal of analytical toxicology, 32(9), 760-762 (2008-11-22)
Zinc as a marker element in the viscera of suspected metal phosphide poisoning has been studied during the present work. Neutron activation analysis (NAA) has been employed to detect and quantify the concentration of zinc in the viscera/stomach portion. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service