17-10189
LentiBrite GFP-LC3 Control Mutant Lentiviral Biosensor
Synonym(s):
Microtubule-associated proteins 1A/1B light chain 3, Autophagy-related protein LC3, Autophagy-related ubiquitin-like modifier LC3, MAP1A/MAP1B light chain 3, Microtubule-associated protein 1 light chain 3
About This Item
Recommended Products
General description
http://www.nature.com/app_notes/nmeth/2012/121007/pdf/an8620.pdf
(Click Here!)
Learn more about the advantages of our LentiBrite Lentiviral Biosensors! Click Here
Biosensors can be used to detect the presence/absence of a particular protein as well as the subcellular location of that protein within the live state of a cell. Fluorescent tags are often desired as a means to visualize the protein of interest within a cell by either fluorescent microscopy or time-lapse video capture. Visualizing live cells without disruption allows researchers to observe cellular conditions in real time.
Lentiviral vector systems are a popular research tool used to introduce gene products into cells. Lentiviral transfection has advantages over non-viral methods such as chemical-based transfection including higher-efficiency transfection of dividing and non-dividing cells, long-term stable expression of the transgene, and low immunogenicity.
EMD Millipore is introducing LentiBrite Lentiviral Biosensors, a new suite of pre-packaged lentiviral particles encoding important and foundational proteins of autophagy, apoptosis, and cell structure for visualization under different cell/disease states in live cell and in vitro analysis.
- Pre-packaged, fluorescently-tagged with GFP & RFP
- Higher efficiency transfection as compared to traditional chemical-based and other non-viral-based transfection methods
- Ability to transfect dividing, non-dividing, and difficult-to-transfect cell types, such as primary cells or stem cells
- Non-disruptive towards cellular function
EMD Millipore’s LentiBrite GFP-LC3-G120A mutant lentiviral particles serve as a negative control alongside GFP-LC3 wild-type (catalog # 17-10193) for live cell analysis of autophagy.
A mutant form of LC3 with the C-terminal glycine changed to alanine (LC3-G120A) is unable to accept the PE modification and fails to translocate to the autophagosome upon induction of autophagy.
EMD Millipore’s LentiBrite GFP-LC3-G120A lentiviral particles serve as a negative control alongside GFP-LC3 wild-type (catalog # 17-10193) for live cell analysis of autophagy.
Application
Imaging:
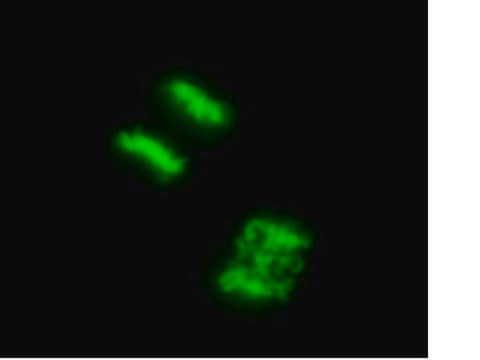
(See Figure 1 in datasheet)
HT-1080 cells were plated in a chamber slide and transduced with lentiviral particles at an MOI of 20 for 24 hours. After media replacement and 48 hours further incubation, cells were either lleft in complete media or incubated for 4 hours in EBSS containing a lysosome inhibitor, to induce autophagy and inhibit lysosomal degradation of autophagosomes. Cells were fixed with formaldehyde and mounted. Images were obtained by oil immersion wide-field fluorescence microscopy. The GFP-LC3 Control Mutant displays a diffuse nuclear and cytosolic distribution in both fed and starved autophagic cells (i.e., no translocation to a punctate cytoplasmic distribution as characteristic of wild-type LC3).
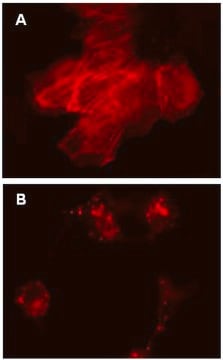
Immunocytochemistry Comparison and Inhibitor Analysis:
(See Figure 2 in datasheet)
Similar to Figure 1 conditions (see datasheet), HeLa cells were plated in a chamber slide and transduced with lentiviral particles at an MOI of 20 for 24 hours. After media replacement and 48 hours further incubation, cells were either left in complete media, incubated for 4 hours in EBSS containing a lysosome inhibitor to induce autophagy and inhibit lysosomal degradation, or incubated as in, with the addition of 5 mM 3-methyladenine (3-MA) as an inhibitor of autophagy. 3-MA does not affect GFP-LC3 Control Mutant localization. Immunocytochemical staining (red) of the same fields of view with a monoclonal antibody against LC3A reveals a similar expression pattern to the mutant protein (green) under fed conditions. Immunostaining of starved cells displays the punctate distribution of endogenous wild-type LC3, while signal following 3-MA treatment is diminished.
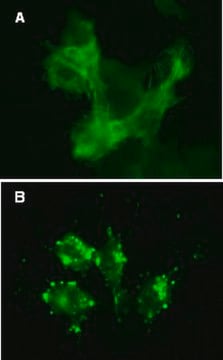
Hard-to-transfect Cell Types:
(See Figure 3 in datasheet)
Primary cell types HUVEC or HuMSC were plated in chamber slides and transduced with lentiviral particles at an MOI of 40 for 24 hours. Subsequent treatments for cells left in complete media or cells incubated in EBSS with lysosome inhibitor, were performed as in Figures 1A and 1B (see datasheet).
For optimal fluorescent visualization, it is recommended to analyze the target expression level within 24-48 hrs after transfection/infection for optimal live cell analysis, as fluorescent intensity may dim over time, especially in difficult-to-transfect cell lines. Infected cells may be frozen down after successful transfection/infection and thawed in culture to retain positive fluorescent expression beyond 24-48 hrs. Length and intensity of fluorescent expression varies between cell lines. Higher MOIs may be required for difficult-to-transfect cell lines.
Apoptosis & Cancer
Neuroscience
Apoptosis - Additional
Neurodegenerative Diseases
Components
One vial containing 25 µL of lentiviral particles at a minimum of 3 x 10E8 infectious units (IFU) per mL.
For lot-specific titer information, please see lot specific “Viral Titer” in the product specifications of the datasheet.
Promoter
EF-1 (Elongation Factor-1)
Multiplicty of Infection (MOI)
MOI = Ratio of # of infectious lentiviral particles (IFU) to # of cells being infected.
Typical MOI values for high transduction efficiency and signal intensity are in the range of 20-40. For this target, some cell types may require lower MOIs (e.g., HeLa, human mesenchymal stem cells (HuMSC)), while others may require higher MOIs (e.g., HT-1080, human umbilical vein endothelial cells (HUVEC), U2OS).
NOTE: MOI should be titrated and optimized by the end user for each cell type and lentiviral target to achieve desired transduction efficiency and signal intensity.
Quality
Physical form
Storage and Stability
Lentivirus is stable for at least 4 months from date of receipt when stored at -80°C. After first thaw, place immediately on ice and freeze in working aliquots at -80°C. Frozen aliquots may be stored for at least 2 months. Further freeze/thaws may result in decreased virus titer and transduction efficiency.
IMPORTANT SAFETY NOTE
Replication-defective lentiviral vectors, such as the 3rd Generation vector provided in this product, are not known to cause any diseases in humans or animals. However, lentiviruses can integrate into the host cell genome and thus pose some risk of insertional mutagenesis. Material is a Risk Group 2 and should be handled under BSL2 controls. A detailed discussion of biosafety of lentiviral vectors is provided in Pauwels, K. et al. (2009). State-of-the-art lentiviral vectors for research use: Risk assessment and biosafety recommendations. Curr. Gene Ther. 9: 459-474.
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Cell based autophagy assays including live cell LC3 GFP/RFP lentiviral biosensors, kits for autophagy detection and autophagy activators and inhibitors.
Cell based autophagy assays including live cell LC3 GFP/RFP lentiviral biosensors, kits for autophagy detection and autophagy activators and inhibitors.
Cell based autophagy assays including live cell LC3 GFP/RFP lentiviral biosensors, kits for autophagy detection and autophagy activators and inhibitors.
Cell based autophagy assays including live cell LC3 GFP/RFP lentiviral biosensors, kits for autophagy detection and autophagy activators and inhibitors.
Related Content
Fluorescent lentiviral particles encoding important GFP/RFP fusion proteins related to autophagy, apoptosis, and cell structure that enables live cell imaging.
Fluorescent lentiviral particles encoding important GFP/RFP fusion proteins related to autophagy, apoptosis, and cell structure that enables live cell imaging.
Fluorescent lentiviral particles encoding important GFP/RFP fusion proteins related to autophagy, apoptosis, and cell structure that enables live cell imaging.
Fluorescent lentiviral particles encoding important GFP/RFP fusion proteins related to autophagy, apoptosis, and cell structure that enables live cell imaging.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service