62262
Ethyl linoleate
technical, ≥65% (GC)
Synonym(s):
Linoleic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
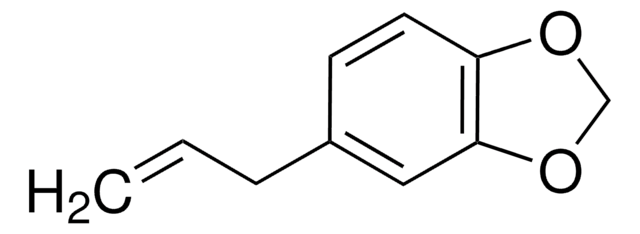
CH3(CH2)3(CH2CH=CH)2(CH2)7COOC2H5
CAS Number:
Molecular Weight:
308.50
Beilstein:
1727827
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
concentration
≥65% (GC)
refractive index
n20/D 1.455 (lit.)
n20/D 1.455
bp
224 °C/17 mmHg (lit.)
density
0.876 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CCCCC\C=C/C\C=C/CCCCCCCC(=O)OCC
InChI
1S/C20H36O2/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22-4-2/h8-9,11-12H,3-7,10,13-19H2,1-2H3/b9-8-,12-11-
InChI key
FMMOOAYVCKXGMF-MURFETPASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl linoleate can undergo auto-oxidation in the presence of the catalyst manganese(II)acetylacetonate.
Application
Ethyl linoleate can be used as a drying agent for alkyd paints. It can also form N2,3-ethenoguanine via reaction with deoxyguanosine.
Biochem/physiol Actions
Ethyl linoleate solated from Oxalis triangularis inhibits forskolin-induced melanogenesis and tyrosinase activity in mouse B16 melanoma cells . This anti-melanogenic effect is mediated by inhibiting cAMP production.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
"Fast autoxidation of ethyl linoleate catalyzed by [Mn (acac) 3] and bipyridine: A possible drying catalyst for alkyd paints"
Gorkum VR, et al.
Inorganic Chemistry, 43(08), 2456-2458 (2004)
"4-Hydroxy-2-nonenal and ethyl linoleate form N 2, 3-ethenoguanine under peroxidizing conditions"
Ham.L J-A, et al.
Chemical Research in Toxicology, 1243-1250 (2000)
Yun Ding et al.
Current biology : CB, 29(7), 1089-1099 (2019-03-19)
It is unclear where in the nervous system evolutionary changes tend to occur. To localize the source of neural evolution that has generated divergent behaviors, we developed a new approach to label and functionally manipulate homologous neurons across Drosophila species.
D L Luthria et al.
Biochimica et biophysica acta, 1213(1), 1-4 (1994-06-23)
In order to determine how dietary linoleate is metabolized, rats were maintained on a chemically defined diet containing 1.6% ethyl linoleate. After 5 weeks the linoleate was replaced by an equal amount of ethyl 9,10,12,13-d4-linoleate. The animals were killed 3
A Murakami et al.
Oncology, 53(5), 386-391 (1996-09-01)
The anti-tumor-promoting activity of 1'-acetoxychavicol acetate (ACA) was examined in a two-stage carcinogenesis experiment in ICR mouse skin using 7,12-dimethylbenz[a]anthracene (0.19 mumol) and 12-O-tetradecanoylphorbol-13-acetate (TPA; 1.6 nmol). Topical application of ACA (160 nmol) markedly reduced the average number of tumors
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service