All Photos(1)
About This Item
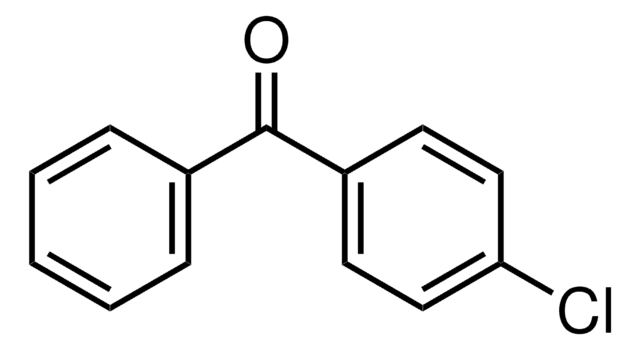
Linear Formula:
ClC6H4COC6H5
CAS Number:
Molecular Weight:
216.66
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
bp
330 °C (lit.)
mp
44-47 °C (lit.)
functional group
chloro
ketone
phenyl
SMILES string
Clc1ccccc1C(=O)c2ccccc2
InChI
1S/C13H9ClO/c14-12-9-5-4-8-11(12)13(15)10-6-2-1-3-7-10/h1-9H
InChI key
VMHYWKBKHMYRNF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Thermodynamics of formation of inclusion complex between 2-chlorobenzophenone and cyclomaltoheptaose (β-cyclodextrin) has been investigated by UV-vis spectroscopy and reversed-phase liquid chromatography. 2-Chlorobenzophenone undergoes reduction in the presence of LiAlH4 and (R)-(-)-2-(2-iso-indolinyl)butan-1-ol to afford the corresponding benzhydrols.
Application
2-Chlorobenzophenone was used in the synthesis of 1-(2-chlorophenyl)isoquinolin-3-yl trifluoromethanesulfonate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Matias I Sancho et al.
Carbohydrate research, 346(13), 1978-1984 (2011-06-07)
A thermodynamic study of the inclusion process between 2-chlorobenzophenone (2ClBP) and cyclomaltoheptaose (β-cyclodextrin, β-CD) was performed using UV-vis spectroscopy, reversed-phase liquid chromatography (RP-HPLC), and molecular modeling (PM6). Spectrophotometric measurements in aqueous solutions were performed at different temperatures. The stoichiometry of
Asymmetric reductions of ketones using lithium aluminium hydride modified with N, N-dialkyl derivatives of (R)-(-)-2-aminobutan-1-ol.
Brown E, et al.
Tetrahedron Asymmetry, 2(5), 339-342 (1991)
Synthesis of N-methyl-N-(1-methylpropyl)-1-(2-chlorophenyl) isoquinoline-3-[11 C] carboxamide ([11 C-carbonyl] PK11195) and some analogues using [11 C] carbon monoxide and 1-(2-chlorophenyl) isoquinolin-3-yl triflate.
Rahman O, et al.
Journal of the Chemical Society. Perkin Transactions 1, 23, 2699-2703 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service