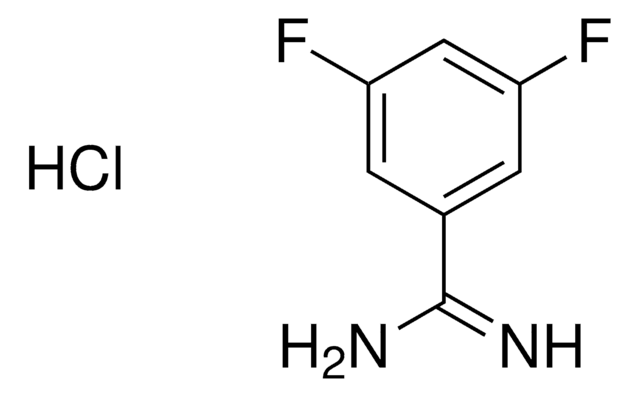
63226
Benzamidine hydrochloride 1 M solution
Synonym(s):
Benzamidine hydrochloride solution, Additive Screening Solution 37/Kit-No 78374
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
storage temp.
2-8°C
SMILES string
Cl.NC(=N)c1ccccc1
InChI
1S/C7H8N2.ClH/c8-7(9)6-4-2-1-3-5-6;/h1-5H,(H3,8,9);1H
InChI key
LZCZIHQBSCVGRD-UHFFFAOYSA-N
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Single-molecule approach to DNA minor-groove association dynamics.
Jorge Bordello et al.
Angewandte Chemie (International ed. in English), 51(30), 7541-7544 (2012-06-16)
Yu Cheng Zhu et al.
Pest management science, 68(5), 692-701 (2012-01-10)
The potential development of resistance to Bacillus thuringiensis (Bt) cotton and surging of non-targeted insects is a major risk in the durability of Bt plant technology. Midgut proteinases are involved in Bt activation and degradation. Proteinase inhibitors may be used
Luca Mannocci et al.
Bioconjugate chemistry, 21(10), 1836-1841 (2010-09-03)
Collections of chemical compounds, individually attached to unique DNA fragments serving as amplifiable identification bar codes, are generally referred to as "DNA-encoded chemical libraries". Such libraries can be used for the de novo isolation of binding molecules against target proteins
Amy Capes et al.
Bioorganic & medicinal chemistry, 20(4), 1607-1615 (2012-01-24)
Quinols have been developed as a class of potential anti-cancer compounds. They are thought to act as double Michael acceptors, forming two covalent bonds to their target protein(s). Quinols have also been shown to have activity against the parasite Trypanosoma
Janet Newman et al.
Journal of computer-aided molecular design, 26(5), 497-503 (2011-12-22)
Part of the latest SAMPL challenge was to predict how a small fragment library of 500 commercially available compounds would bind to a protein target. In order to assess the modellers' work, a reasonably comprehensive set of data was collected
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service