696188
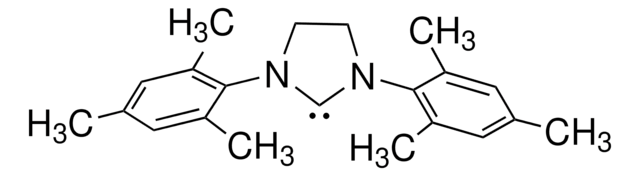
1,3-Bis(2,4,6-trimethylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene
97%
Synonym(s):
1,3-Bis(2,4,6-trimethylphenyl)imidazol-2-ylidene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C21H24N2
CAS Number:
Molecular Weight:
304.43
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
reaction suitability
reagent type: catalyst
mp
140 °C
storage temp.
−20°C
SMILES string
Cc1cc(C)c(N2[C]N(C=C2)c3c(C)cc(C)cc3C)c(C)c1
InChI
1S/C21H24N2/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6/h7-12H,1-6H3
InChI key
JCYWCSGERIELPG-UHFFFAOYSA-N
Application
1,3-Bis(2,4,6-trimethylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene (IMes) is a nucleophilic N-heterocyclic carbene (NHC) ligand.
It can be used to synthesize:
IMes is also used as an ancillary ligand in Pd-catalyzed Suzuki-Miyaura cross-coupling reaction between aryl chlorides or aryl triflates and arylboronic acids.
It can be used to synthesize:
- IMes ligated-rhodium complex as a catalyst for the selective hydrogenation of substituted aryl and heteroaryl boronate esters to cis-substituted borylated cycloalkanes.
- IMes/ruthenium complex (Cp*Ru(IMes)Cl)(Cp* =η5-C5Me3) as a catalyst for olefin ring closing metathesis reaction.
IMes is also used as an ancillary ligand in Pd-catalyzed Suzuki-Miyaura cross-coupling reaction between aryl chlorides or aryl triflates and arylboronic acids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gilles Schnee et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(49), 17959-17972 (2015-10-21)
The present contribution reports experimental and theoretical mechanistic investigations on a normal-to-abnormal (C2-to-C4-bonded) NHC rearrangement processes occurring with bulky group 13 metal NHC adducts, including the scope of such a reactivity for Al compounds. The sterically congested adducts (nItBu)MMe3 (nItBu=1,3-di-tert-butylimidazol-2-ylidene;
Suzuki- Miyaura cross-coupling reactions mediated by palladium/imidazolium salt systems
Grasa GA, et al.
Organometallics, 21(14), 2866-2873 (2002)
Hydrogenation of (Hetero) aryl Boronate Esters with a Cyclic (Alkyl)(amino) carbene-Rhodium Complex: Direct Access to cis-Substituted Borylated Cycloalkanes and Saturated Heterocycles
Ling L, et al.
Angewandte Chemie (International Edition in English), 58(20), 6554-6558 (2019)
Olefin metathesis-active ruthenium complexes bearing a nucleophilic carbene ligand
Huang J, et al.
Journal of the American Chemical Society, 121(12), 2674-2678 (1999)
Thi Kim Hoang Trinh et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(39), 9242-9252 (2019-04-26)
In the search of smarter routes to control the conditions of N-heterocyclic carbene (NHCs) formation, a two-component air-stable NHC photogenerating system is reported. It relies on the irradiation at 365 nm of a mixture of 2-isopropylthioxanthone (ITX) with 1,3-bis(mesityl)imidazoli(ni)um tetraphenylborate. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![2-Mesityl-5-methylimidazo[1,5-a]pyridinium chloride 97%](/deepweb/assets/sigmaaldrich/product/structures/495/055/5d86d2cc-b538-4586-9e2c-9e0d870826a7/640/5d86d2cc-b538-4586-9e2c-9e0d870826a7.png)



![Chloro[1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene]gold(I) 95%](/deepweb/assets/sigmaaldrich/product/structures/154/609/370330fe-5c15-47b6-ad13-ea3dc87099d6/640/370330fe-5c15-47b6-ad13-ea3dc87099d6.png)