All Photos(1)
About This Item
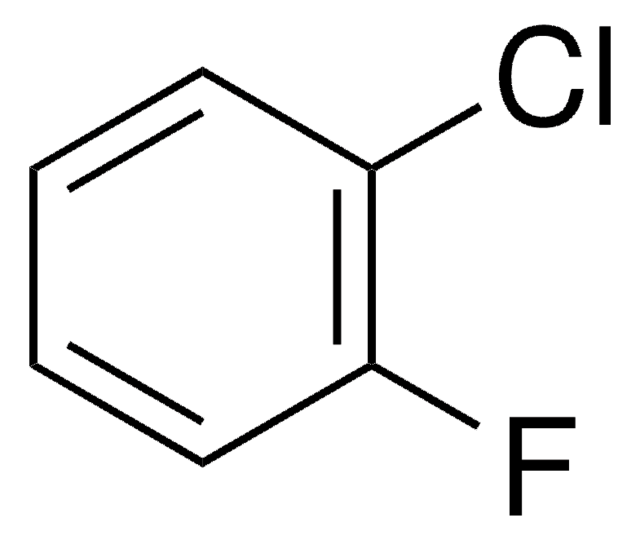
Linear Formula:
ClC6H3F2
CAS Number:
Molecular Weight:
148.54
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.475 (lit.)
bp
127 °C (lit.)
density
1.353 g/mL at 25 °C (lit.)
SMILES string
Fc1ccc(Cl)c(F)c1
InChI
1S/C6H3ClF2/c7-5-2-1-4(8)3-6(5)9/h1-3H
InChI key
AJCSNHQKXUSMMY-UHFFFAOYSA-N
Application
1-Chloro-2,4-difluorobenzene was used in the preparation of series of benzonorbornadienes. It was also used in the preparation of difluoroarenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
91.4 °F - closed cup
Flash Point(C)
33 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K C Caster et al.
The Journal of organic chemistry, 66(9), 2932-2936 (2001-04-28)
This report details the synthesis of several benzonorbornadienes by Diels--Alder cycloaddition of cyclopentadiene derivatives with substituted benzyne intermediates, which were generated by low-temperature metal--halogen exchange of halobenzenes. General conditions were developed, allowing synthesis of most benzonorbornadienes described herein at the
Jason T Manka et al.
The Journal of organic chemistry, 69(6), 1967-1971 (2004-04-03)
Aryldiazo substituents were used in nucleophilic aromatic substitution reactions of halogens. The Ph-N=N- group activates ortho fluorine atoms toward alkylthiolation under mild conditions. In contrast, the Me(2)N-C(6)H(4)-N=N- group has virtually no activation effect in nucleophilic aromatic substitution, and serves as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service