137995
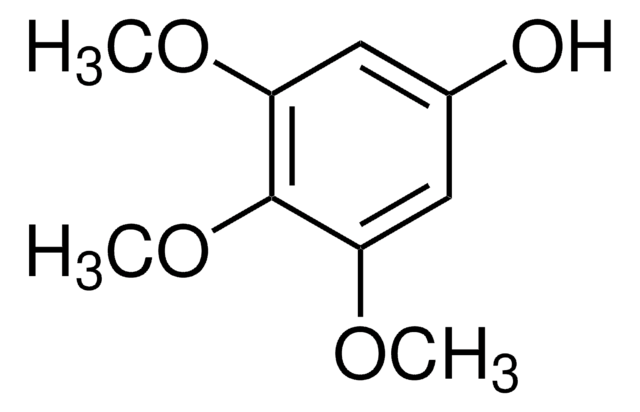
1,2,3-Trimethoxybenzene
98%
Synonym(s):
Pyrogallol trimethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H3(OCH3)3
CAS Number:
Molecular Weight:
168.19
Beilstein:
1910422
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
chunks
bp
241 °C (lit.)
mp
43-47 °C (lit.)
density
1.112 g/mL at 25 °C (lit.)
SMILES string
COc1cccc(OC)c1OC
InChI
1S/C9H12O3/c1-10-7-5-4-6-8(11-2)9(7)12-3/h4-6H,1-3H3
InChI key
CRUILBNAQILVHZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,2,3-Trimethoxybenzene on condensation with 2,4-diamino-5-(hydroxymethyl)pyrimidine yields 2,4-diamino-5-(2,3,4-trimethoxybenzyl)pyrimidine.
Application
1,2,3-Trimethoxybenzene was used to study the effect of solvent on photoinduced electron-transfer reactions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Romelo Gibe et al.
Chemical communications (Cambridge, England), (14)(14), 1550-1551 (2002-08-23)
Nicholas reactions of the p-phenyl linked bis(propargyl acetate) complex (3a) with electron rich arenes give cyclophanetetrayne complexes (5). The use of bis(propargyl ether) complex (8) with (3a) allows formation of a mixed cyclophanetetrayne complex (9), and in addition gives retro-Nicholas/intramolecular
Zhangjie Shi et al.
Journal of the American Chemical Society, 126(19), 5964-5965 (2004-05-13)
A gold-catalyzed cyclialkylation of electron-rich arenes with tethered epoxides afforded 3-chromanols stereospecifically.
A Stuart et al.
Journal of medicinal chemistry, 26(5), 667-673 (1983-05-01)
A new route to 2,4-diamino-5-(4-hydroxybenzyl)pyrimidines has been developed that involves the condensation of 2,4-diamino-5-(hydroxymethyl)pyrimidine with phenols in acidic medium. The use of phenol and its 2,6-dialkyl derivatives produces 5-(4-hydroxybenzyl)pyrimidines exclusively. However, 2,6-dimethoxyphenol produces a mixture of 5-(3-hydroxy-2,4-dimethoxybenzyl)- and 5-(4-hydroxy-3,5-dimethoxybenzyl)pyrimidines. The
Solvent effects on photoinduced electron-transfer reactions.
Niwa T, et al.
The Journal of Physical Chemistry, 97(46), 11960-11964 (1993)
Ramjee Kandel et al.
Dalton transactions (Cambridge, England : 2003), 48(33), 12512-12521 (2019-08-01)
Phosphaamidine metal complexes Rh2Cl2[Ph2PC(Ph)[double bond, length as m-dash]NPh]2μ-CO (1), RuCl2[Ph2PC(Ph)[double bond, length as m-dash]N(Ph)]2 (2), [Rh{iPr2PC(Ph)[double bond, length as m-dash]NiPr}(COD)]BF4 (3), and RuCl2[iPr2PC(Ph)[double bond, length as m-dash]NiPr](DMSO)2 (4) are prepared by combining phosphaamidines Ph2P-C(Ph)[double bond, length as m-dash]NPh and iPr2P-C(Ph)[double
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service