86872
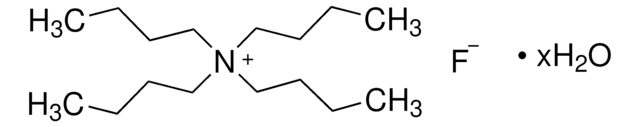
Tetrabutylammonium fluoride trihydrate
≥97.0% (NT)
Synonym(s):
TBAF
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[CH3(CH2)3]4NF · 3H2O
CAS Number:
Molecular Weight:
315.51
Beilstein:
3761900
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (NT)
form
crystals
mp
62-63 °C (lit.)
functional group
amine
SMILES string
O.O.O.[F-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.FH.3H2O/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;;;;/h5-16H2,1-4H3;1H;3*1H2/q+1;;;;/p-1
InChI key
VEPTXBCIDSFGBF-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Tetrabutylammonium fluoride trihydrate is a mild base used in reactions like aldol-type condensation reactions, Michael-type reactions, ring-opening reactions. Its is also used as a promoter in cross-coupling reactions and cyclization of carbocycles and heterocycles.
Application
Reactant for:
Preparation of deprotecting agents in preparation of cellulose derivatives
Synthesis of lipophilic peptides for DNA transfections in vivo
Dehydrobromination reactions
Preparation of deprotecting agents in preparation of cellulose derivatives
Synthesis of lipophilic peptides for DNA transfections in vivo
Dehydrobromination reactions
Tetrabutylammonium fluoride trihydrate can be used as a base:
It can be used to catalyze ethynylation of quinolines and isoquinolines using calcium carbide in aqueous N,N-dimethylacetamide.
- For the dehydrobromination of vinyl bromides to terminal acetylenes.
- In the conversion of 1,1-dibromo-1-alkenes to terminal alkynes via Corey–Fuchs reaction.
- In Hiyama cross-coupling reaction of aryl and heteroaryl chlorides with aryltrialkoxysilanes in the presence of a palladium catalyst.
It can be used to catalyze ethynylation of quinolines and isoquinolines using calcium carbide in aqueous N,N-dimethylacetamide.
Other Notes
Reagent for the cleavage of silyl ethers and other silyl protecting groups; Catalyst for various reactions with silicon compounds; Use as a base in organic synthesis; Instability of anhydrous TBAF
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrabutylammonium fluoride-induced dehydrobromination of vinyl bromides to terminal acetylenes
Okutani M and Mori Y
Tetrahedron Letters, 48(39), 6856-6859 (2007)
T.W. Greene et al.
Protective Groups in Organic Synthesis (1991)
An Efficient Method for the Production of Terminal Alkynes from 1, 1-Dibromo-1-alkenes and its Application in the Total Synthesis of Natural Product Dihydroxerulin
Liu S, et al.
advanced synthesis and catalysis, 357(2-3), 553-560 (2015)
G. Majetich et al.
The Journal of Organic Chemistry, 51, 1745-1745 (1986)
An Efficient Method for the Production of Terminal Alkynes from 1, 1-Dibromo-1-alkenes and its Application in the Total Synthesis of Natural Product Dihydroxerulin
Liu S, et al.
Advanced Synthesis & Catalysis, 357(2-3), 553-560 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service