740683
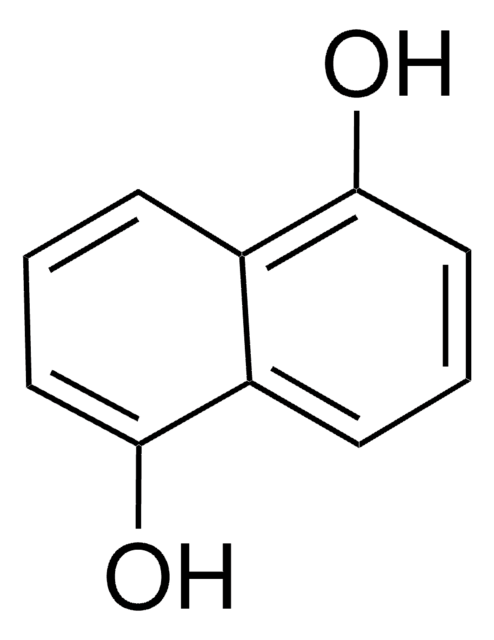
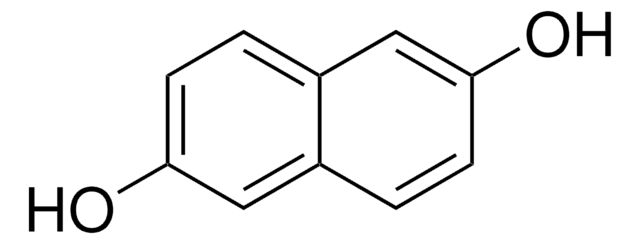
1,8-Dihydroxynaphthalene
95%
Synonym(s):
1,8-Naphthalenediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H8O2
CAS Number:
Molecular Weight:
160.17
Beilstein:
2044947
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
137-143 °C
storage temp.
2-8°C
SMILES string
Oc1cccc2cccc(O)c12
InChI
1S/C10H8O2/c11-8-5-1-3-7-4-2-6-9(12)10(7)8/h1-6,11-12H
InChI key
OENHRRVNRZBNNS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,8-Dihydroxynaphthalene (DHN) can be used as:
- An intermediate in the preparation of benzo analogs of spiromamakone A.
- A starting material to synthesize naphthopyran derivatives.
- An intermediate in the total synthesis of palmarumycin CP17 analogs.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E S Jacobson et al.
Infection and immunity, 63(12), 4944-4945 (1995-12-01)
1,8-Dihydroxynaphthalene melanin in Wangiella dermatitidis and Alternaria alternata was titrated in vivo with the oxidants permanganate, hypochlorite, and H2O2. Melanized strains neutralized more oxidant and withstood higher concentrations of permanganate and hypochlorite than albino strains did. H2O2 killing required 1,000-fold
Pigment biosynthesis and virulence.
A A Brakhage et al.
Contributions to microbiology, 2, 205-215 (1999-10-16)
R Romero-Martinez et al.
Infection and immunity, 68(6), 3696-3703 (2000-05-19)
Sporothrix schenckii is a human pathogen that causes sporotrichosis, an important cutaneous mycosis with a worldwide distribution. It produces dark-brown conidia, which infect the host. We found that S. schenckii synthesizes melanin via the 1,8-dihydroxynaphthalene pentaketide pathway. Melanin biosynthesis in
Synthesis of spiromamakone a benzo analogues via double oxa-michael addition of 1, 8-dihydroxynaphthalene
Tsukamoto H, et al.
Organic Letters, 18, 4848-4851 (2016)
H F Tsai et al.
Journal of bacteriology, 180(12), 3031-3038 (1998-06-11)
Aspergillus fumigatus, an important opportunistic pathogen which commonly affects neutropenic patients, produces conidia with a bluish-green color. We identified a gene, alb1, which is required for conidial pigmentation. The alb1 gene encodes a putative polyketide synthase, and disruption of alb1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service