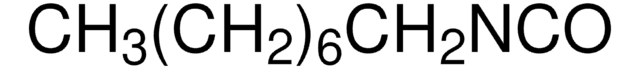All Photos(1)
About This Item
Linear Formula:
CH3(CH2)5NCO
CAS Number:
Molecular Weight:
127.18
Beilstein:
1751836
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.421 (lit.)
bp
162-164 °C (lit.)
density
0.873 g/mL at 25 °C (lit.)
functional group
amine
isocyanate
SMILES string
CCCCCCN=C=O
InChI
1S/C7H13NO/c1-2-3-4-5-6-8-7-9/h2-6H2,1H3
InChI key
ANJPRQPHZGHVQB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hexyl isocyanate has been investigated for its antigenicity in the guinea pig animal model of hapten-specific respiratory hypersensitivity. Polymerization of n-hexyl isocyanate using rare earth tris(2,6-di-tert-butyl-4-methylphenolate) [Ln(OAr)3] as initiator has been studied.
Application
Hexyl isocyanate may be employed for the synthesis of following:
- rigid-rod, helical isocyanate-based macromonomers
- rod-coil-rod triblock copolymers with 2-vinylpyridine, by living anionic polymerization
- chiral poly(n-hexyl isocyanate) (PHIC) macromonomers, by living anionic polymerization
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 3 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
138.2 °F - closed cup
Flash Point(C)
59 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Garret M Miyake et al.
Journal of the American Chemical Society, 134(34), 14249-14254 (2012-08-16)
The synthesis of rigid-rod, helical isocyanate-based macromonomers was achieved through the polymerization of hexyl isocyanate and 4-phenylbutyl isocyanate, initiated by an exo-norbornene functionalized half-titanocene complex. Sequential ruthenium-mediated ring-opening metathesis polymerization of these macromonomers readily afforded well-defined brush block copolymers, with
Chiroptical Properties of Graft Copolymers Containing Chiral Poly (n-hexyl isocyanate) as a Side Chain.
Shah PN, et al.
Macromolecules, 44(20), 7917-7925 (2011)
Synthesis and Self-Assembly Studies of Amphiphilic Poly (n-hexyl isocyanate)-b lock-poly (2-vinylpyridine)-b lock-poly (n-hexyl isocyanate) Rod-Coil-Rod Triblock Copolymer.
Rahman M, et al.
Macromolecules, 39(15), 5009-5014 (2006)
Boer Liu et al.
Molecules (Basel, Switzerland), 26(15) (2021-08-08)
This work reveals the influence of pendant hydrogen bonding strength and distribution on self-assembly and the resulting thermomechanical properties of A-AB-A triblock copolymers. Reversible addition-fragmentation chain transfer polymerization afforded a library of A-AB-A acrylic triblock copolymers, wherein the A unit
Pulmonary hypersensitivity to hexyl isocyanate-ovalbumin aerosol in guinea pigs.
M H Karol et al.
Toxicology and applied pharmacology, 51(1), 73-80 (1979-10-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service