All Photos(1)
About This Item
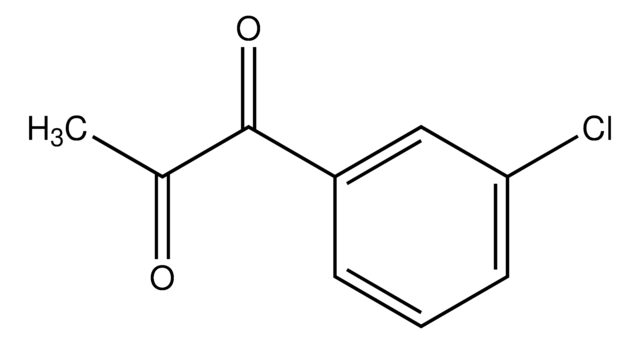
Linear Formula:
ClC6H4COC2H5
CAS Number:
Molecular Weight:
168.62
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
124 °C/14 mmHg (lit.)
mp
45-47 °C (lit.)
SMILES string
CCC(=O)c1cccc(Cl)c1
InChI
1S/C9H9ClO/c1-2-9(11)7-4-3-5-8(10)6-7/h3-6H,2H2,1H3
InChI key
PQWGFUFROKIJBO-UHFFFAOYSA-N
Related Categories
General description
Influence of solvents and temperature on the yield and enantioselectivity of the phenylation of 3′-chloropropiophenone has been investigated.
Application
3′-Chloropropiophenone can be used as a reactant to synthesize:
- (S)-3-chloro-1-phenylpropanol via bio-catalyzed asymmetric reduction method.
- 1-(3-Chlorophenyl)-1-phenyl-1-propanol by phenylation with diphenylzinc in the presence of dihydroxy bis(sulfonamide) ligand.
- (S)-Dapoxetine, a selective serotonin reuptake inhibitor.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enhancing asymmetric reduction of 3-chloropropiophenone with immobilized Acetobacter sp. CCTCC M209061 cells by using deep eutectic solvents as cosolvents
Xu Pei, et al.
ACS sustainable chemistry & engineering, 3(4), 718-724 (2015)
Asymmetric reduction of (S)-3-chloro-1-phenylpropanol from 3-chloropropiophenone by preheated immobilized Candida utilis
Yang Gen-Sheng, et al.
Biotechnology Letters, 12, 1879-1883 (2009)
Asymmetric total synthesis of (S)-dapoxetine
Kim Sun Joo, et al.
Tetrahedron Letters, 53(28), 3680-3682 (2012)
Celina García et al.
Organic letters, 5(20), 3641-3644 (2003-09-26)
[reaction: see text] The catalytic asymmetric addition of phenyl groups from diphenylzinc to ketones is reported. The catalyst, generated from a dihydroxy bis(sulfonamide) ligand and titanium tetraisopropoxide, gives good to excellent enantioselectivities with a range of substrates.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service