130036
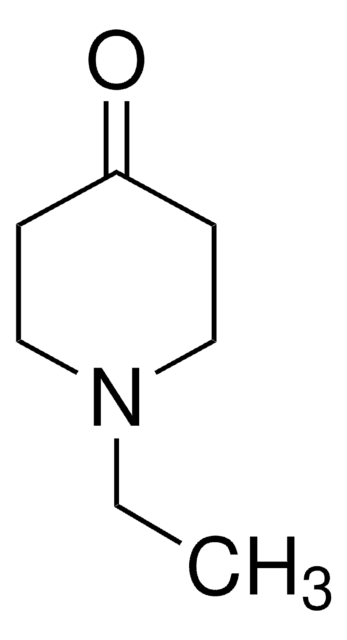
N-Methyl-4-piperidone
97%
Synonym(s):
1-Methyl-4-piperidinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11NO
CAS Number:
Molecular Weight:
113.16
Beilstein:
106924
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
reg. compliance
suitable for FDA C-010.02
Assay
97%
form
liquid
density
0.92 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CN1CCC(=O)CC1
InChI
1S/C6H11NO/c1-7-4-2-6(8)3-5-7/h2-5H2,1H3
InChI key
HUUPVABNAQUEJW-UHFFFAOYSA-N
Application
N-Methyl-4-piperidone can be used as a reactant to prepare:
- Spiropiperidine rings by reacting with malononitrile and electrophiles or Michael acceptors.
- (3E,5E)-1-Methyl-3,5-bis(phenylmethylene)-4-piperidinone by reacting with benzaldehyde via Michael addition, followed by intramolecular O-cyclization/elimination sequential reactions.
- N,N′-Dimethylbispidinone by utilizing a double Mannich condensation method.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Analogs of sparteine. I. Reexamination of the reaction of N-methyl-4-piperidone with formaldehyde and methylamine. Revised synthesis of N, N'-dimethylbispidinone
Smissman EE, et al.
The Journal of Organic Chemistry, 40, 251-252 (1975)
Novel route to spiropiperidines using N-methyl-4-piperidone, malononitrile and electrophiles
Lakshmi NV, et al.
Tetrahedron Letters, 53, 1282-1286 (2012)
Analogs of sparteine. I. A reexamination of the reaction of n-methyl-4-piperidone with formaldehyde and methylamine. A revised synthesis of n,n'-dimethylbispidinone.
E E Smissman et al.
The Journal of organic chemistry, 40(2), 251-252 (1975-01-24)
A facile tandem Michael addition/O-cyclization/elimination route to novel chromeno [3, 2-c] pyridines
Sumesh RV, et al.
Molecular Diversity, 19, 233-249 (2015)
Bin-Rong Yao et al.
European journal of medicinal chemistry, 167, 187-199 (2019-02-17)
To get new anti-hepatoma agents with anti-inflammatory activity and hypotoxicity, a series of dissymmetric pyridyl-substituted 3,5-bis(arylidene)-4-piperidones (BAPs, 25-82) were designed and synthesized. Many of them exhibited potential anti-hepatoma properties against human hepatocellular carcinoma cell lines (HepG2, QGY-7703, SMMC-7721) and hypotoxicity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service