ABK0007
HercBridge ELISA Kit
Synonyme(s) :
Protein Conformational Array Herceptin Biosimilar, Protein Conformational Array Trastuzumab
About This Item
Produits recommandés
Forme d'anticorps
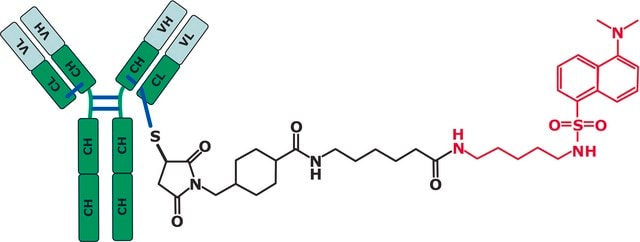
IgG fraction of antiserum
Espèces réactives
human (Human IgG)
Conditions d'expédition
wet ice
Température de stockage
2-8°C
Description générale
Application
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 1
Listes réglementaires
Les listes réglementaires sont principalement fournies pour les produits chimiques. Seules des informations limitées peuvent être fournies ici pour les produits non chimiques. L'absence d'indication signifie qu'aucun des composants n'est répertorié. Il incombe à l'utilisateur de s'assurer de l'utilisation sûre et légale du produit.
EU REACH Annex XVII (Restriction List)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Monoclonal antibodies (mAbs) lead biologics growth, predicted $125 billion worldwide sales by 2020, emphasizing their therapeutic significance.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique