21872
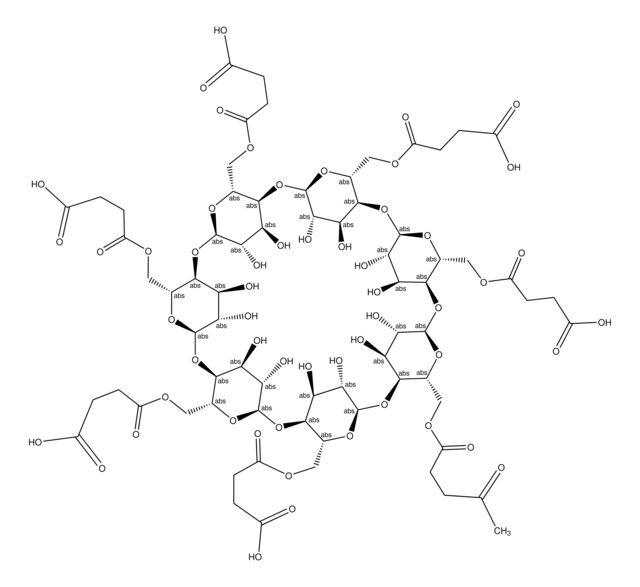
(2-Carboxyethyl)-β-cyclodextrin sodium salt
About This Item
Produits recommandés
Forme
solid
Activité optique
[α]20/D +129±6°, c = 1% in H2O
Impuretés
≤10% acidic form
~5% water
Solubilité
H2O: 50 mg/mL, clear, colorless
Température de stockage
room temp
Description générale
Application
- As a chiral selector for the enantioseparation of cinacalcet impurities[2] and fenamiphos pesticide[3] by capillary zone electrophoresis and 31P nuclear magnetic resonance spectroscopy (31P NMR), respectively.
- To form an inclusion complex with carvacrol for subsequent use in the antibacterial multilayer construction with chitosan, loaded onto poly(L-lactic acid) (PLLA).[1]
Remarque sur l'analyse
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique