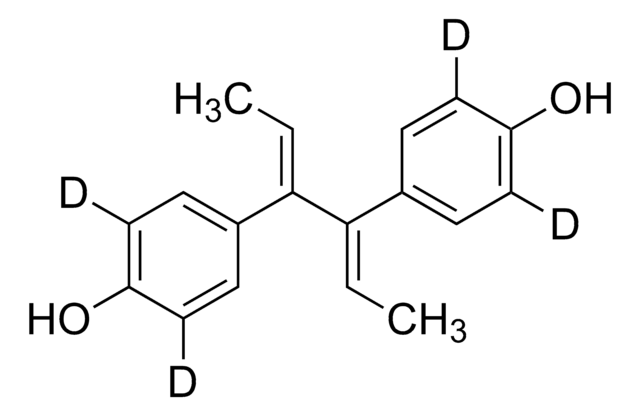
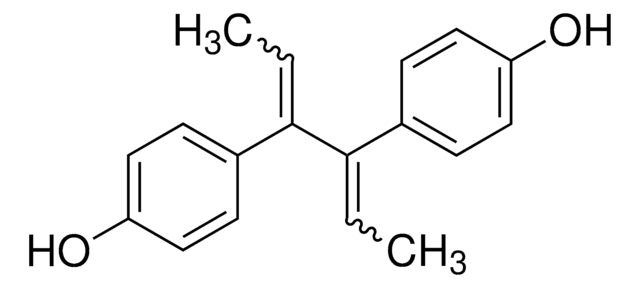
H7753
Hexestrol
analytical standard
Synonyme(s) :
4,4′-(1,2-diéthyléthylene)diphénol
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Essai
≥98%
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
forensics and toxicology
pharmaceutical (small molecule)
veterinary
Format
neat
Chaîne SMILES
CC[C@H]([C@H](CC)c1ccc(O)cc1)c2ccc(O)cc2
InChI
1S/C18H22O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h5-12,17-20H,3-4H2,1-2H3/t17-,18+
Clé InChI
PBBGSZCBWVPOOL-HDICACEKSA-N
Informations sur le gène
human ... ESR1(2099)
rat ... Esr1(24890)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Liaison
Produits recommandés
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Carc. 1B
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 2
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique