919888
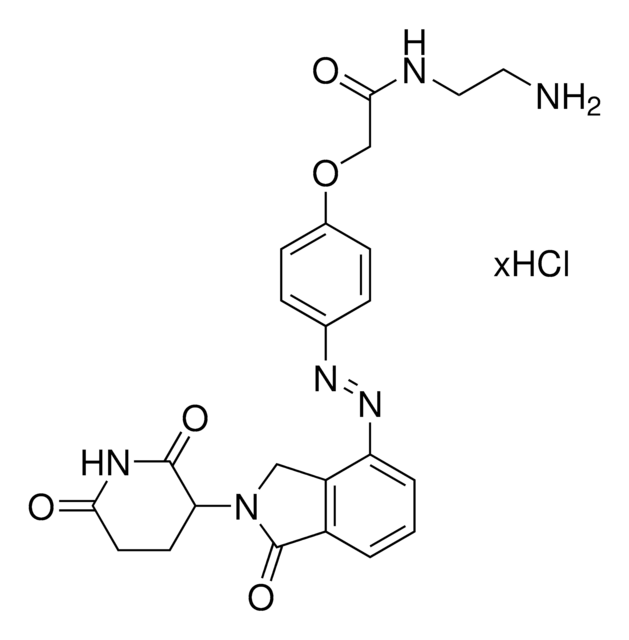
Thalidomide-Photoswitch3-NH2 hydrochloride
≥95%
Synonyme(s) :
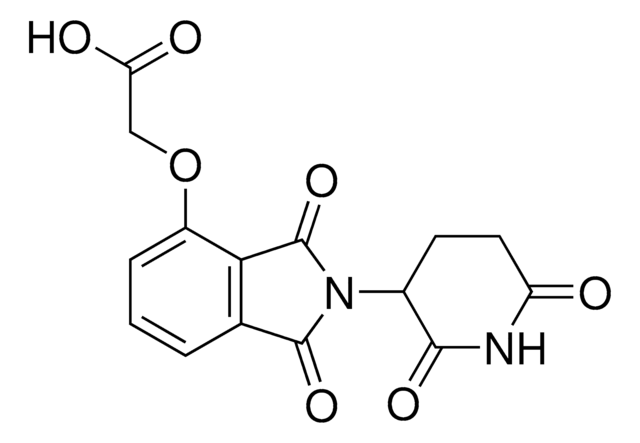
(E)-N-(4-((4-Aminophenyl)diazenyl)phenyl)-2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetamide hydrochloride, Photoswitchable protein degrader building block for PROTAC®
About This Item
Produits recommandés
ligand
thalidomide
Niveau de qualité
Pureté
≥95%
Forme
solid
Pertinence de la réaction
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
Groupe fonctionnel
amine
Température de stockage
2-8°C
Chaîne SMILES
O=C1N(C2C(NC(CC2)=O)=O)C(C3=C1C=CC=C3OCC(NC4=CC=C(/N=N/C5=CC=C(N)C=C5)C=C4)=O)=O.Cl
Catégories apparentées
Application
Suggested wavelengths for photoswitching:
- Switch to cis isomer: 390 nm (380-400 nm)
- Switch to trans isomer (thermally more stable isomer): >450 nm
Browse our full offering of degrader building blocks that streamlines the synthesis of degrader libraries.
Learn more:
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Portal: Building PROTAC Degraders for Targeted Protein Degradation
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Autres remarques
Informations légales
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique