907278
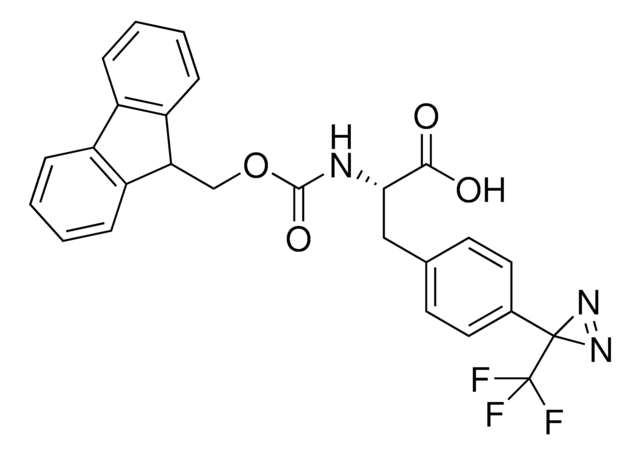
H-L-Photo-leucine HCl
≥98%
Synonyme(s) :
(S)-2-Amino-3-(3-methyl-3H-diazirin-3-yl)propanoic acid hydrochloride, (S)-2-Amino-3-(3H-diazirin-3-yl)butanoic acid hydrochloride, Diazirine amino acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Produits recommandés
Pureté
≥98%
Forme
solid
Capacité de réaction
reaction type: solution phase peptide synthesis
Disponibilité
available only in USA
Application(s)
peptide synthesis
Température de stockage
−20°C
Application
Autres remarques
for generation of homogeneous conjugates from wild-type antibodies
Mechanistic studies of a small-molecule modulator of SMN2 splicing
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Direct Interaction between an Allosteric Agonist Pepducin and the Chemokine Receptor CXCR4
Photo-leucine and photo-methionine allow identification of protein-?protein interactions in living cells
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Self-react. C
Code de la classe de stockage
5.2 - Organic peroxides and self-reacting hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique