S1071
α-Synuclein A53T human
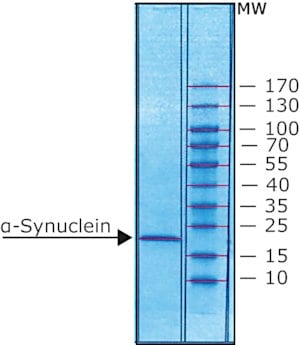
recombinant, expressed in E. coli, N-terminal histidine tagged, ≥90% (SDS-PAGE), lyophilized powder
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
Assay
≥90% (SDS-PAGE)
form
lyophilized powder
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
Gene Information
human ... SNCA(6622)
General description
α-Synuclein is mapped to human chromosome 4q22.1. It is an intrinsically disordered protein with N-terminal imperfect repeats (KTKEGV), a central NAC crucial for aggregation and an acidic rich flexible C-terminal region.α-Synuclein is present in the Lewy bodies (LBs) and Lewy neurites (LNs).
Application
α-Synuclein A53T human has been used to treat mesencephalic neuronal and stimulate microglial cells prior to reactive oxygen species (ROS) measurement, immunohistochemistry and imaging studies.
Biochem/physiol Actions
α-Synuclein (α-Syn) interacts with toll-like receptor 2 (TLR2) and mediates interleukin-1β (IL-1β) synthesis.
A point mutation in the α-synuclein gene, A53T (Ala53-Thr), is linked to familial Parkinson′s disease. Mice expressing A53T human α-synuclein, but not wild-type or the A30P variants, develop adult-onset neurodegenerative disease with a progressive motoric dysfunction leading to death.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mehmet Ozansoy et al.
Molecular neurobiology, 47(2), 460-465 (2012-11-28)
Parkinson's disease (PD) is the second most common neurodegenerative disorder, defined by the presence of resting tremor, muscular rigidity, bradykinesia, and postural instability. PD is characterized by the progressive loss of dopaminergic neurons within the substantia nigra pars compacta of
Nathan Meyer et al.
ACS central science, 8(4), 441-448 (2022-05-05)
The detection to α-synuclein (αS) assemblies as a biomarker of synucleinopathies is an important challenge for further development of an early diagnosis tool. Here, we present proof of concept real-time fast amyloid seeding and translocation (RT-FAST) based on a nanopipette
A short motif in the N-terminal region of α-synuclein is critical for both aggregation and function.
Ciaran P A Doherty et al.
Nature structural & molecular biology, 27(3), 249-259 (2020-03-12)
Aggregation of human α-synuclein (αSyn) is linked to Parkinson's disease (PD) pathology. The central region of the αSyn sequence contains the non-amyloid β-component (NAC) crucial for aggregation. However, how NAC flanking regions modulate αSyn aggregation remains unclear. Using bioinformatics, mutation
Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53? Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice
Lee MK, et al.
Proceedings of the National Academy of Sciences, 99(13), 8968-8973 (2002)
Yan Zhou et al.
Molecular neurodegeneration, 11, 28-28 (2016-04-17)
α-Synuclein (α-Syn), a pathological hallmark of Parkinson's disease (PD), has been recognized to induce the production of interleukin-1β in a process that depends, at least in vitro, on nod-like receptor protein 3 (NLRP3) inflammasome in monocytes. However, the role of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service