L6638
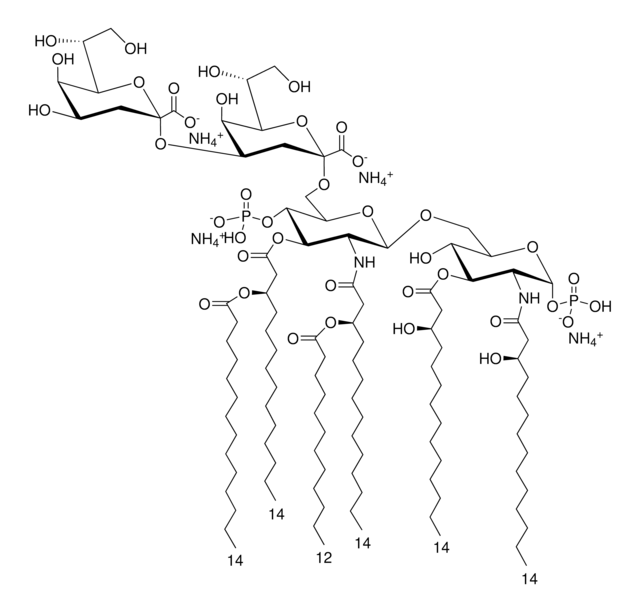
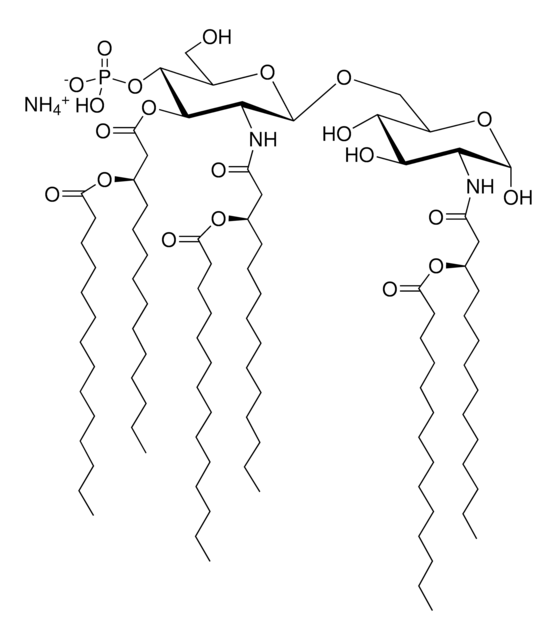
Lipid A, monophosphoryl from Escherichia coli F583 (Rd mutant)
lyophilized powder
Synonym(s):
E. coli Monophosphoryl Lipid A
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Escherichia coli (F583 Rd mutant)
Quality Level
form
lyophilized powder
impurities
<0.2% Ketodeoxyoctonate (KDO)
shipped in
ambient
storage temp.
2-8°C
Related Categories
Biochem/physiol Actions
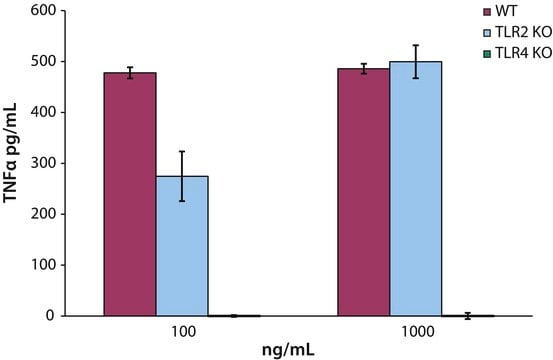
Lipid A molecules compose the lipid membrane anchoring core components of endotoxins produced by Gram-negative bacteria. Lipid A molecules induce immune responses. Structually, lipid A molecules are composed of two glucosamine unites with varied, species dependent, fatty acyl chain number and identity and degree of phosphorylation.
Lipid A, monophosphoryl from Escherichia coli F583 may be used in comparative assessment of the antigenicity of specific structures within different LPA molecules and analogues.
Lipid A, monophosphoryl from Escherichia coli F583 may be used in comparative assessment of the antigenicity of specific structures within different LPA molecules and analogues.
Lipid A has immunostimulatory adjuvant activity including macrophage, T-cell, and B-cell activation. Research has shown Lipid A has greater Th1 than Th2 stimulation.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vincent J Venditto et al.
Clinical and vaccine immunology : CVI, 21(8), 1086-1093 (2014-05-30)
Broadly neutralizing monoclonal antibodies (bNAbs) 2F5 and 4E10 bind to the membrane proximal external region (MPER) of gp41 and also cross-react with phospholipids. In this study, we investigated if chemical modifications on the MPER adjacent to 2F5 and 4E10 epitopes
Vincent J Venditto et al.
Clinical and vaccine immunology : CVI, 20(1), 39-45 (2012-11-02)
The inability to generate broadly neutralizing antibody (bnAb) responses to the membrane proximal external region (MPER) of HIV-1 gp41 using current vaccine strategies has hampered efforts to prevent the spread of HIV. To address this challenge, we investigated a novel
Mark B Stoddard et al.
Clinical and vaccine immunology : CVI, 17(1), 98-107 (2009-11-20)
Bacterial endotoxin interacts with the human immune system via complex immunological pathways. The evaluation of endotoxicity is important in the development of safe vaccines and immunomodulatory therapeutics. The Limulus amebocyte lysate (LAL) assay is generally accepted by the FDA for
Douglas S Watson et al.
Clinical and vaccine immunology : CVI, 18(2), 289-297 (2010-12-17)
Particulate delivery systems enhance antibody responses to subunit antigens. However, covalent attachment of protein antigens can disrupt protein structure and mask critical epitopes, altering the antibody response to the antigen. In this report, we evaluate noncovalent metal chelation via nitrilotriacetic
Hong Zhao et al.
PLoS pathogens, 10(4), e1004009-e1004009 (2014-04-12)
Metarhizium robertsii is a plant root colonizing fungus that is also an insect pathogen. Its entomopathogenicity is a characteristic that was acquired during evolution from a plant endophyte ancestor. This transition provides a novel perspective on how new functional mechanisms
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service