42604
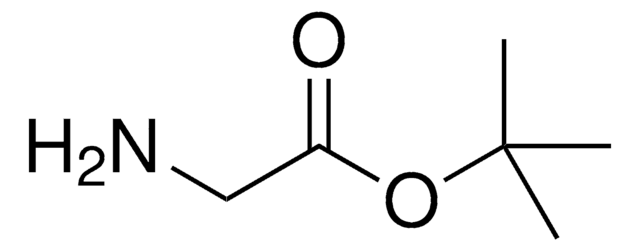
Glycine tert-butyl ester hydrochloride
puriss., ≥99.0% (AT)
Synonym(s):
tert-Butyl aminoacetate hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NCH2CO2C(CH3)3 · HCl
CAS Number:
Molecular Weight:
167.63
Beilstein:
3989048
MDL number:
UNSPSC Code:
12352200
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
puriss.
Quality Level
Assay
≥99.0% (AT)
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
141-143 °C (lit.)
solubility
H2O: 0.1 g/mL, clear, colorless
application(s)
peptide synthesis
SMILES string
Cl.CC(C)(C)OC(=O)CN
InChI
1S/C6H13NO2.ClH/c1-6(2,3)9-5(8)4-7;/h4,7H2,1-3H3;1H
InChI key
OSWULUXZFOQIRU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S Sarhan et al.
Arzneimittel-Forschung, 34(6), 687-690 (1984-01-01)
The synergistic amplification of the anticonvulsant effects of direct and indirect GABA agonists by glycine has previously been demonstrated. We show in the present report that the anticonvulsant effect of vinyl GABA, a GABA-T (4-aminobutyrate: 2-oxoglutarate aminotransferase) inhibitor with antiepileptic
M Nakoji et al.
Organic letters, 3(21), 3329-3331 (2001-10-12)
[reaction: see text]. A chiral phase-transfer catalyst has been applied to the asymmetric allylation of the tert-butyl glycinate-benzophenone Schiff base with various allylic acetates for the first time to give the allylated products in good yields and with comparable to
Baptiste Thierry et al.
Molecular diversity, 9(4), 277-290 (2005-11-29)
Cross-linked polystyrene-bound and poly(ethylene glycol)-bound phase-transfer catalysts as well as homopolymers of cinchona alkaloid derivatives have been synthesised. Both soluble and insoluble polymers have been investigated. The enantioselective alkylation of N-diphenyl methylene glycine t-butyl ester has been successfully carried out
Ming-Qing Hua et al.
Chemical communications (Cambridge, England), 47(5), 1631-1633 (2010-12-01)
An efficient, catalytic, diastereo- and enantioselective conjugate addition of N-(diphenylmethylene)glycine tert-butyl ester to β-aryl substituted enones was realized in the presence of 1 mol% of newly desired dinuclear N-spiro-ammonium salts, affording functionalized α-amino acid derivatives in 57-98% yields with high
V De Filippis et al.
Biochemistry, 37(39), 13507-13515 (1998-09-30)
Hirudin is the most potent and specific inhibitor of thrombin, a key enzyme in the coagulation process existing in equilibrium between its procoagulant (fast) and anticoagulant (slow) form. In a previous study, we described the solid-phase synthesis of a Trp3
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service