W509000
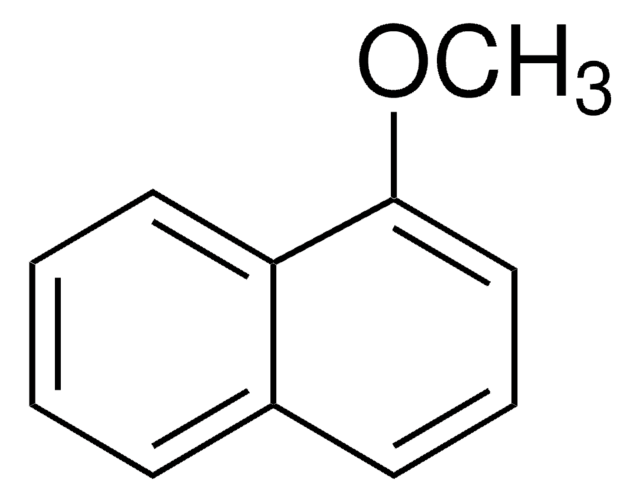
2-Methoxynaphthalene
≥99%
Synonym(s):
Methyl 2-naphthyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C10H7OCH3
CAS Number:
Molecular Weight:
158.20
Beilstein:
1859408
EC Number:
MDL number:
UNSPSC Code:
12164502
PubChem Substance ID:
Flavis number:
4.074
Recommended Products
biological source
synthetic
grade
Kosher
Assay
≥99%
bp
274 °C (lit.)
mp
70-73 °C (lit.)
density
1.064 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
SMILES string
COc1ccc2ccccc2c1
InChI
1S/C11H10O/c1-12-11-7-6-9-4-2-3-5-10(9)8-11/h2-8H,1H3
InChI key
LUZDYPLAQQGJEA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Features and Benefits
Intensely sweet, floral, mild orange blossom
Other Notes
Download our Flavors and Fragrances Catalog to view our entire product line.
Subscribe to our Newsletter to keep up to date on our latest Flavors and Fragrances offerings.
Subscribe to our Newsletter to keep up to date on our latest Flavors and Fragrances offerings.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P Di Gennaro et al.
Biotechnology and bioengineering, 93(3), 511-518 (2005-09-30)
The bioconversion of naphthalene to the 1,2-dihydro-1,2-dihydroxy derivative was performed in good yield using an Escherichia coli recombinant strain carrying Pseudomonas fluorescens N3 dioxygenase. However, the efficiency of such transformation is affected by many process parameters, and their optimization is
G M Whited et al.
Bioorganic & medicinal chemistry, 2(7), 727-734 (1994-07-01)
2-Methoxynaphthalene was subjected to biooxidation by whole cells of six organisms: Pseudomonas putida F39/D containing toluene dioxygenase, Escherichia coli JM109(pDTG601), containing recombinant toluene dioxygenase from Pp F39/D, Pseudomonas sp. NCIB 9816/11, containing naphthalene dioxygenase. E. coli JM109(pDTG141), containing recombinant naphthalene
H Widén et al.
Food additives and contaminants, 22(7), 681-692 (2005-07-16)
Mineral water and soft drinks with a perceptible off-odour were analysed to identify contaminants originating from previous misuse of the refillable polyethylene terephthalate (PET) bottle. Consumers detected the off-odour after opening the bottle and duly returned it with the remaining
Mingzhang Gao et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 68(3), 459-465 (2010-01-12)
Carbon-11-labeled piperidine ring of N-[omega-(6-methoxynaphthalen-1-yl)alkyl] derivatives were first designed and synthesized as new selective PET sigma(1) receptor probes. The target tracers were prepared by O-[(11)C]methylation of their corresponding phenolic hydroxyl precursors using [(11)C]CH(3)OTf under basic conditions and isolated by a
R R Price et al.
Journal of microencapsulation, 10(2), 215-222 (1993-04-01)
Many natural products that exhibit biocidal activity have poor solubility in water. In order to explore the prolonged delivery of these compounds from microtubules we have utilized 2-methoxynaphthalene as a model to elucidate release characteristics of hydrophobic compounds entrapped in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service