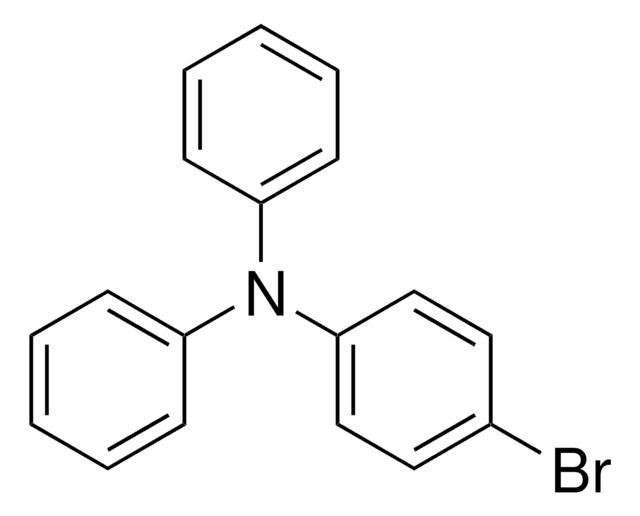
T81604
Triphenylamine
98%
Synonym(s):
N,N-Diphenylaniline, N,N-Diphenylbenzenamine
About This Item
Recommended Products
Assay
98%
bp
347-348 °C (lit.)
mp
124-128 °C (lit.)
SMILES string
c1ccc(cc1)N(c2ccccc2)c3ccccc3
InChI
1S/C18H15N/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
InChI key
ODHXBMXNKOYIBV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Photoinitiators for Hydrogel Formation: Triphenylamine derivatives are used as novel D-pi-A hemicyanine dyes, acting as effective photoinitiators for in situ hydrogel formation and DLP printing, expanding their applications in biomedical engineering and 3D printing technologies (Du et al., 2024).
- Supramolecular Photosensitizers: A supramolecular construct based on triphenylamine and pyrazine demonstrates aggregation-induced emission properties, enhancing the efficiency of photooxidation reactions. This development offers potential improvements in photodynamic therapy and environmental applications (Dong et al., 2024).
- Highly Efficient OLEDs: Triphenylamine is integral in synthesizing new phenanthro[9,10-d]oxazole-based fluorophores with hybridized local and charge-transfer characteristics. These materials are crucial for developing blue non-doped OLEDs with minimal efficiency roll-off, significant for advanced display technologies (Xie et al., 2024).
- Memory Device Applications: Modifications in donor end caps in N-heteroaromatic systems containing triphenylamine were explored for binary-to-ternary WORM memory conversion, contributing to advancements in memory storage technology (Gayathri et al., 2024).
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service