All Photos(1)
About This Item
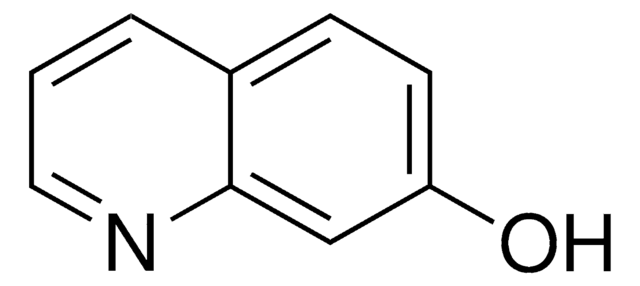
Empirical Formula (Hill Notation):
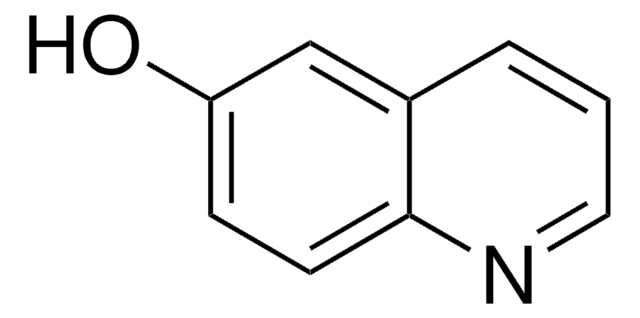
C9H7NO
CAS Number:
Molecular Weight:
145.16
Beilstein:
2900
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
200-202 °C (lit.)
SMILES string
Oc1ccnc2ccccc12
InChI
1S/C9H7NO/c11-9-5-6-10-8-4-2-1-3-7(8)9/h1-6H,(H,10,11)
InChI key
PMZDQRJGMBOQBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Quinolinol (4-quinolone) is a quinolone compound which forms the core moiety of antibacterials such as norfloxacin, nalidixic acid, ciprofloxacin and cinoxacin.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Óscar M Bautista-Aguilera et al.
International journal of molecular sciences, 21(11) (2020-06-04)
In this communication, we report the synthesis and cholinesterase (ChE)/monoamine oxidase (MAO) inhibition of 19 quinolinones (QN1-19) and 13 dihydroquinolinones (DQN1-13) designed as potential multitarget small molecules (MSM) for Alzheimer's disease therapy. Contrary to our expectations, none of them showed
Josip Podobnik et al.
Biomolecules, 10(11) (2020-11-19)
Juvenile delinquency is related to several biological factors, yet very few vulnerability biomarkers have been identified. Previous data suggest that the enzyme monoamine oxidase B (MAO-B) influences several personality traits linked to the propensity to engage in delinquent behavior. Building
Methods of analysis of 4-quinolone antibacterials.
Belal F, et al.
Talanta, 50(4), 765-786 (1999)
A Morinan et al.
Journal of pharmacological methods, 13(3), 213-223 (1985-06-01)
A fluorimetric assay for monamine oxidase that uses kynuramine as the substrate is described. This method is more economic, rapid, reproducible, and sensitive than other similar procedures. Its validity has been confirmed by the study of the subcellular distribution, the
Eduardo Borges de Melo
European journal of medicinal chemistry, 45(12), 5817-5826 (2010-10-23)
Two multivariate studies, a PCA-SAR and a PLS-QSAR, of 3-aryl-4-hydroxyquinolin-2(1H)-one derivatives described as type I fatty acid synthase (FAS) inhibitors, are presented in this work. The variable selection was performed with the Fisher's weight and Ordered Predictors Selection (OPS) algorithm
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service