576662
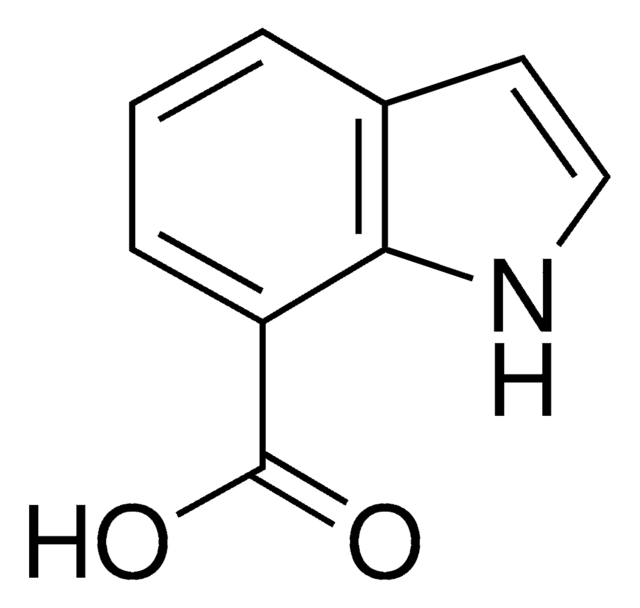
Indole-6-carboxylic acid
97%
Synonym(s):
6-Carboxyindole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H7NO2
CAS Number:
Molecular Weight:
161.16
Beilstein:
123991
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
249-253 °C (lit.)
SMILES string
OC(=O)c1ccc2cc[nH]c2c1
InChI
1S/C9H7NO2/c11-9(12)7-2-1-6-3-4-10-8(6)5-7/h1-5,10H,(H,11,12)
InChI key
GHTDODSYDCPOCW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Reactant for preparation of D-glutamic acid-based inhibitors of E. coli MurD ligase
- Reactant for preparation of indolylindazoles and indolylpyrazolopyridines as interleukin-2 inducible T cell kinase inhibitors
- Reactant for preparation of amide conjugates with ketoprofen, as inhibitors of Gli1-mediated transcription in Hedgehog pathway
- Reactant for preparation of piperazine-bisamide analogs as human growth hormone secretagogue receptor antagonists for treatment of obesity
- Reactant for preparation of pyridinyl carboxylates via esterification with chlorohydroxypyridine as SARS-CoV 3CL proinhibitors
- Reactant for preparation of (indolecarbonyl)-D-phenylglycinamide amides as factor Xa inhibitors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thomas Heine et al.
Applied biochemistry and biotechnology, 181(4), 1590-1610 (2016-11-11)
The enantioselective epoxidation of styrene and related compounds by two-component styrene monooxygenases (SMOs) has targeted these enzymes for development as biocatalysts. In the present work, we prepare genetically engineered fusion proteins that join the C-terminus of the epoxidase (StyA) to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service