All Photos(1)
About This Item
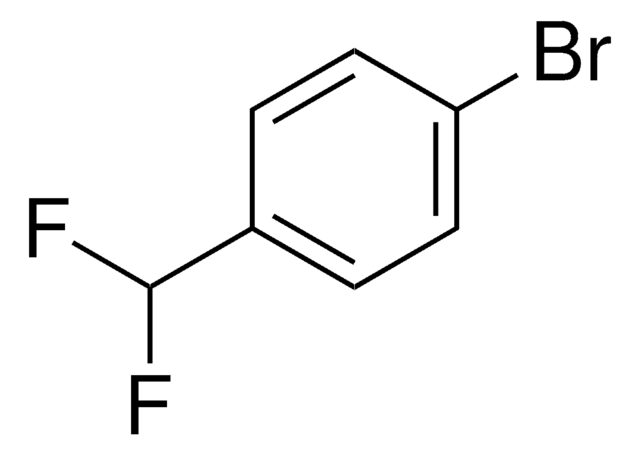
Linear Formula:
Br(CH3)C6H3NH2
CAS Number:
Molecular Weight:
186.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.6030 (lit.)
bp
105-107 °C/205 mmHg (lit.)
density
1.4780 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
Cc1cccc(Br)c1N
InChI
1S/C7H8BrN/c1-5-3-2-4-6(8)7(5)9/h2-4H,9H2,1H3
InChI key
LDUCMSVRKKDATH-UHFFFAOYSA-N
Application
2-Bromo-6-methylaniline can be used as a reactant to synthesize:
- Alkyl substituted bromoindazole building blocks by Pd-catalyzed Suzuki coupling reaction with various vinyl boronic acids.
- Difluoropyridoindole via Pd-catalyzed intramolecular Heck reaction with difluoropiperidinone.
- 4-Methyl-N-phenyl-1H-benzo[d]imidazol-2-amine by one-pot Cu-catalyzed domino C-N cross-coupling reaction with iodobenzene and thiourea.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
215.1 °F - closed cup
Flash Point(C)
101.7 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Copper-promoted one-pot approach: Synthesis of benzimidazoles
Boddapati SN, et al.
Molecules (Basel), 25(8), 1788-1788 (2020)
Novel synthesis of 4, 4-difluoropyrido [4, 3-b] indoles via intramolecular Heck reaction
Madaiah M, et al.
Tetrahedron Letters, 54(11), 1424-1427 (2013)
Synthesis and evaluation of indazole based analog sensitive Akt inhibitors
Okuzumi T, et al.
Molecular Biosystems, 6(8), 1389-1402 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service