392588
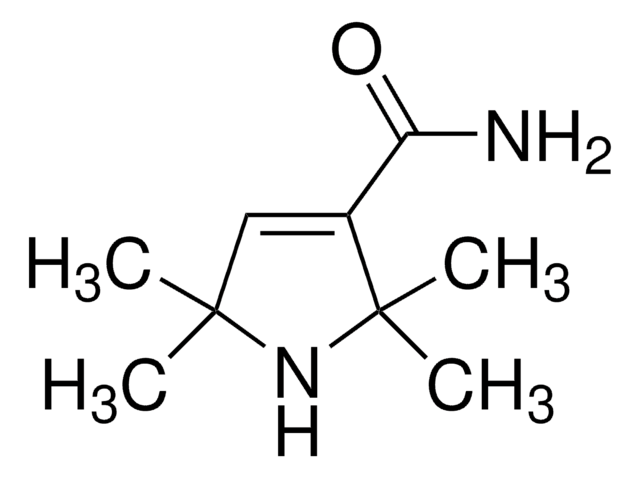
2,2,5,5-Tetramethyl-3-pyrrolidinecarboxamide
98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H18N2O
CAS Number:
Molecular Weight:
170.25
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
128-131 °C (lit.)
SMILES string
CC1(C)CC(C(N)=O)C(C)(C)N1
InChI
1S/C9H18N2O/c1-8(2)5-6(7(10)12)9(3,4)11-8/h6,11H,5H2,1-4H3,(H2,10,12)
InChI key
POAGFQOGFRYOFM-UHFFFAOYSA-N
General description
2,2,5,5-Tetramethyl-3-pyrrolidinecarboxamide (2,2,5,5-Tetramethylpyrrolidine-3-carboxamide) is a carboxamide of hydrogenated pyrrole derivative. Its synthesis by the hydrogenation of 2,2,5,5-tetramethyl-3-pyrroline-3-carboxamide has been reported. The antiarrhythmic activity of some of the derivatives of 2,2,5,5-tetramethylpyrrolidine-3-carboxamide has been evaluated.
Application
2,2,5,5-Tetramethyl-3-pyrrolidinecarboxamide may be used in the synthesis of its nitroxide, 3-carbamoyl-2,2,5,5-tetramethyl-1-pyrrolidinyloxy free radical.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yaws CL.
The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, 260-260 (2015)
Nitroxide free radicals in the hydrogenated pyrrole series.
Rozantsev EG, et al.
Russian Chemical Bulletin, 15(4), 638-641 (1966)
O H Hankovszky et al.
Journal of medicinal chemistry, 29(7), 1138-1152 (1986-07-01)
N-(omega-Aminoalkyl)-2,2,5,5-tetramethyl-3-pyrroline- or -pyrrolidine-3-carboxamides were acylated on the primary amino group of the side chain by means of reactive acid derivatives (acid chlorides, activated esters, phthalic anhydrides, phthalimide, 2-alkyl-4H-3,1-benzoxazin-4-ones) or they were alkylated by forming the Schiff bases and subsequent sodium
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service