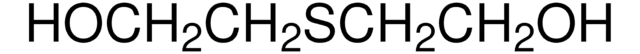235334
3,6-Dithia-1,8-octanediol
97%
Synonym(s):
2,2′-(Ethylenedithio)diethanol, Lindlar Catalyst Poison
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HO(CH2)2S(CH2)2S(CH2)2OH
CAS Number:
Molecular Weight:
182.30
Beilstein:
1739193
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
bp
170 °C/0.5 mmHg (lit.)
mp
63-64 °C (lit.)
SMILES string
OCCSCCSCCO
InChI
1S/C6H14O2S2/c7-1-3-9-5-6-10-4-2-8/h7-8H,1-6H2
InChI key
PDHFSBXFZGYBIP-UHFFFAOYSA-N
Related Categories
General description
3,6-Dithia-1,8-octanediol is a secondary sulfur-based catalyst poison.
Application
3,6-Dithia-1,8-octanediol has been used:
- as exogeneous chelator to evaluate a membrane-permeable copper-selective fluorescent sensor for imaging of kinetically labile copper pools
- in asymmetric total synthesis of (+)-6-epi-castanospermine and polyhydroxylated alkaloid
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Liuchun Yang et al.
Proceedings of the National Academy of Sciences of the United States of America, 102(32), 11179-11184 (2005-08-03)
Copper is an essential micronutrient that plays a central role for a broad range of biological processes. Although there is compelling evidence that the intracellular milieu does not contain any free copper ions, the rapid kinetics of copper uptake and
Asymmetric Total Synthesis of (+)-6-epi-Castanospermine by the Stereoselective Formation of a syn, anti Acetylenic 2-Amino-1, 3-diol Stereotriad.
Louvel J, et al.
European Journal of Organic Chemistry, 2010(15), 2921-2926 (2010)
Eric J Stoner et al.
The Journal of organic chemistry, 68(23), 8847-8852 (2003-11-08)
Functionalized erythromycin 9-oxime derivatives are 6-O-allylated under mild conditions using substituted allyl tert-butyl carbonates under palladium(0) catalysis. This allylation works well where traditional ether-forming protocols function poorly. Allyl tert-butyl carbonates provide higher yields in this reaction than lesser substituted carbonates
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service