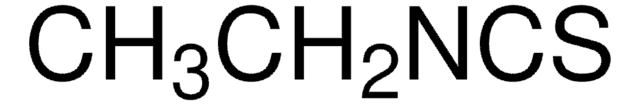All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H10N2O2S
CAS Number:
Molecular Weight:
186.23
Beilstein:
139617
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
92-94 °C (lit.)
SMILES string
CCOC(=O)Cc1csc(N)n1
InChI
1S/C7H10N2O2S/c1-2-11-6(10)3-5-4-12-7(8)9-5/h4H,2-3H2,1H3,(H2,8,9)
InChI key
SHQNGLYXRFCPGZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl 2-aminothiazole-4-acetate is an organic ligand and possess strong coordination ability and display diverse coordination modes due to the presence of N, O coordination atoms.
Application
Ethyl 2-aminothiazole-4-acetate was used in the synthesis of:
- bis (2-aminothiazole-4-acetato)aquazinc(II)
- dichloridobis[ethyl 2-(2-amino-1, 3-thiazol-4-yl) acetate-κ2O,N3]cadmium
- novel heteroaryl-containing benzamide derivatives
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
365.0 °F - closed cup
Flash Point(C)
185 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lai-Jun Zhang et al.
Acta crystallographica. Section E, Structure reports online, 68(Pt 6), m788-m789 (2012-06-22)
The asymmetric unit of the title compound, [CdCl(2)(C(7)H(10)N(2)O(2)S)(2)], contains two complex molecules with similar configurations. The Cd(II) atoms are each six-coordinated by two thiazole N and two carbonyl O atoms from the 2-(2-amino-1,3-thiazol-4-yl)acetate ligand, and by two Cl(-) anions in
Kaapjoo Park et al.
Bioorganic & medicinal chemistry, 22(7), 2280-2293 (2014-03-05)
Novel heteroaryl-containing benzamide derivatives were synthesized and screened using an in vitro assay measuring increases in glucose uptake and glucokinase activity stimulated by 10mM glucose in rat hepatocytes. From a library of synthesized compounds, 3-(4-methanesulfonylphenoxy)-N-[1-(2-methoxy-ethoxymethyl)-1H-pyrazol-3-yl]-5-(3-methyl pyridin-2-yl)-benzamide (19e) was identified as
Lai-Jun Zhang et al.
Acta crystallographica. Section E, Structure reports online, 65(Pt 12), m1517-m1517 (2009-01-01)
In the title compound, [Zn(C(5)H(5)N(2)O(2)S)(2)(H(2)O)], the central Zn atom (2 site symmetry) is five-coordinated by two N and three O atoms [Zn-N = 2.047 (3) Å, Zn-O = 2.099 (2) and 1.974 (4) Å] in a distorted square-pyramidal geometry. Besides one O atom from a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service