All Photos(1)
About This Item
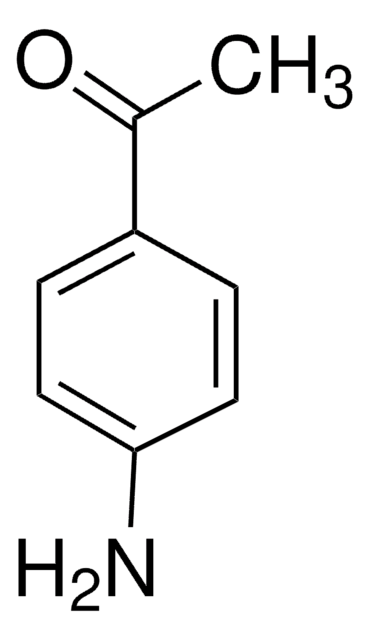
Linear Formula:
CH3COC6H4NH2 · HCl
CAS Number:
Molecular Weight:
171.62
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
285-289 °C (lit.)
functional group
ketone
SMILES string
Cl.CC(=O)c1ccccc1N
InChI
1S/C8H9NO.ClH/c1-6(10)7-4-2-3-5-8(7)9;/h2-5H,9H2,1H3;1H
InChI key
APTPPYQXBFFQHZ-UHFFFAOYSA-N
Application
2′-Aminoacetophenone hydrochloride was used in the synthesis of α-benzamidoacetophenone and indazoles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chloramphenicol1 (Chloromycetin). VI. A Synthetic Approach.
Long LM and Troutman HD.
Journal of the American Chemical Society, 71(7), 2469-2472 (1949)
The Preparation of Indazoles Via Metal Free Intramolecular Electrophilic Amination of 2-Aminophenyl Ketoximes.
M Counceller C, et al.
Organic Syntheses, 33-41 (2012)
Andrew P Worth et al.
Methods in molecular biology (Clifton, N.J.), 930, 125-162 (2012-10-23)
In this chapter, a range of computational tools for applying QSAR and grouping/read-across methods are described, and their integrated use in the computational assessment of genotoxicity is illustrated through the application of selected tools to two case-study compounds-2-amino-9H-pyrido[2,3-b]indole (AαC) and
Quantitative analysis of 2-aminoacetophenone in off-flavored wines by stable isotope dilution assay.
B Dollmann et al.
Journal of AOAC International, 79(2), 583-586 (1996-03-01)
Isotope dilution analysis was used to quantitate 2-aminoacetophenone in wines exhibiting the so-called untypical aging off-flavor. d3-Aminoacetophenone was synthesized and used as isotopomeric internal standard. The method of quantitation was verified by several model experiments. In the off-flavored wines studied
Meenu Kesarwani et al.
PLoS pathogens, 7(8), e1002192-e1002192 (2011-08-11)
A significant number of environmental microorganisms can cause serious, even fatal, acute and chronic infections in humans. The severity and outcome of each type of infection depends on the expression of specific bacterial phenotypes controlled by complex regulatory networks that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service