About This Item
Recommended Products
vapor density
3.72 (vs air)
vapor pressure
1 mmHg ( 20 °C)
Assay
99%
form
(Liquid, Solid, or Crystalline Solid)
autoignition temp.
1038 °F
expl. lim.
1 %
1.1 %, 150 °F
bp
202 °C (lit.)
mp
32-34 °C (lit.)
density
1.034 g/mL at 25 °C (lit.)
SMILES string
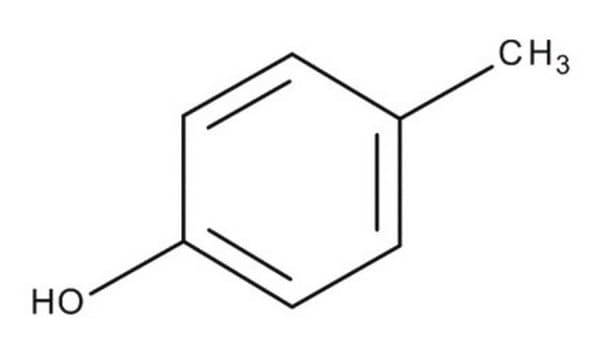
Cc1ccc(O)cc1
InChI
1S/C7H8O/c1-6-2-4-7(8)5-3-6/h2-5,8H,1H3
InChI key
IWDCLRJOBJJRNH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Detoxification of sewage sludge by natural attenuation and implications for its use as a fertilizer on agricultural soils.: This article discusses the role of p-Cresol in the detoxification processes of sewage sludge, considering its implications for safe agricultural use, addressing environmental and health concerns (Mazzeo DEC et al., 2016).
- Characterization of livestock odors using steel plates, solid-phase microextraction, and multidimensional gas chromatography-mass spectrometry-olfactometry.: This research characterizes the complex odors of livestock environments, highlighting the role of p-Cresol in odor profiles, which could help improve management practices and mitigate odor emissions (Bulliner EA et al., 2006).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point(F)
186.8 °F - closed cup
Flash Point(C)
86 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service