Z153192
Cochranes molecular models
minit, classroom set with 5 texts, biochemistry
Synonym(s):
chemistry model sets, molecular model sets
About This Item
Recommended Products
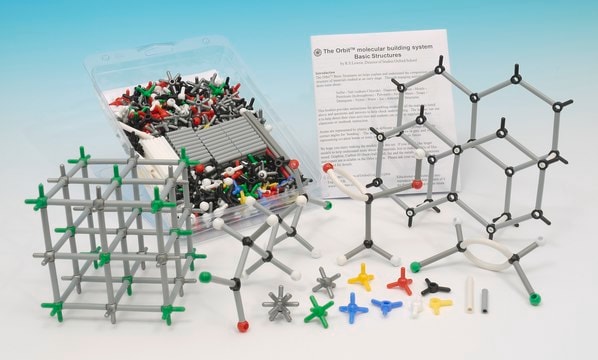
description
Scale: 2 cm = 0.1 nanometer (1 Å), Minit 3 cm = 0.1 nanometer (1 Å), Orbit
feature
no. of atoms 1,370
no. of bonds 1,860
manufacturer/tradename
Cochranes 0078
Looking for similar products? Visit Product Comparison Guide
General description
Scale: 2 cm = 0.1 nanometer (1 A), Minit
ATOMS:
- 1-prong: Hydrogen, white (300), Oxygen, red (50), Sulfur, yellow (20), Chlorine, green (20)
- 2-prong linear: Hydrogen, white (100), Oxygen, red (100)
- 2-prong 100°: Sulfur, yellow (20)
- 2-prong 110°: Nitrogen, blue (20), Oxygen, red (100)
- 2-prong 120°: Nitrogen, blue (25)
- 3-prong 108°, 120°, 132°: Carbon, black (25)
- 3-prong 108°, 126°, 126°: Carbon, black (25)
- 3-prong 114°, 123°, 123°: Carbon, black (100), Nitrogen, blue (120)
- 3-prong 120°: Carbon, black (100), Nitrogen, blue (50)
- 4-prong tetrahedral: Carbon, black (150), Nitrogen, blue (20), Phosphorous, purple (25), Metal, silver (10)
- 6-prong octahedral: Iron, grey (10)
- Peptide link (12)
BONDS:
- Straw, green, 2.5 cm (320)
- Straw, green, 2 cm (640)
- Straw, green, 1.5 cm (720)
- Straw, white, 3.5 cm (160)
- Straw, mixed set/26 (5)
- St v flexible white, 2.5 cm (15)
Please see Technical Information Bulletin AL-183 for a listing of these items, available on our website at sigma-aldrich.com
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Use our handy VSEPR chart to find the 3-D geometric VSEPR shapes of molecules and ions and learn about VSEPR theory and shapes.
Use our handy VSEPR chart to find the 3-D geometric VSEPR shapes of molecules and ions and learn about VSEPR theory and shapes.
Use our handy VSEPR chart to find the 3-D geometric VSEPR shapes of molecules and ions and learn about VSEPR theory and shapes.
Use our handy VSEPR chart to find the 3-D geometric VSEPR shapes of molecules and ions and learn about VSEPR theory and shapes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service