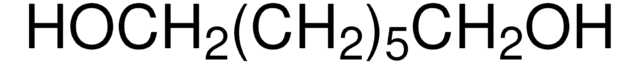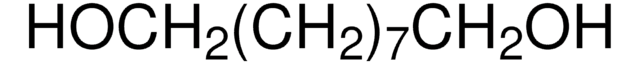O3303
1,8-Octanediol
98%
Synonym(s):
Octamethylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HO(CH2)8OH
CAS Number:
Molecular Weight:
146.23
Beilstein:
1633499
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
172 °C/20 mmHg (lit.)
mp
57-61 °C (lit.)
SMILES string
OCCCCCCCCO
InChI
1S/C8H18O2/c9-7-5-3-1-2-4-6-8-10/h9-10H,1-8H2
InChI key
OEIJHBUUFURJLI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,8-Octanediol can undergo:
- Polycondensation with citric acid to form biodegradable poly(1,8-octanediol citrate)(POC) crosslinked bioelastomer which can be blended with various additives.
- Fischer esterification with dicarboxylic acids to form diol-based macromers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
248.0 °F - closed cup
Flash Point(C)
120 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and Characterization of Diol-Based Unsaturated Polyesters: Poly (diol fumarate) and Poly (diol fumarate-co-succinate).
Tatara AM, et al.
Biomacromolecules, 18(6), 1724-1735 (2017)
Kai-Hee Huong et al.
International journal of biological macromolecules, 116, 217-223 (2018-05-04)
Long carbon chain alkanediols are used in the production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)], however these substrates possess high toxicity towards bacterial cells. This study demonstrated the effective utilisation of a long carbon chain alkanediol, namely 1,8-octanediol, to enhance the yield and
Development of poly (1, 8-octanediol citrate)/chitosan blend films for tissue engineering applications.
Zeimaran E, et al.
Carbohydrate Polymers, 175, 618-627 (2017)
Alexander M Tatara et al.
Biomacromolecules, 18(6), 1724-1735 (2017-05-11)
In this work, we describe the synthesis and characterization of variants of poly(diol fumarate) and poly(diol fumarate-co-succinate). Through a Fischer esterification, α,ω-diols and dicarboxylic acids were polymerized to form aliphatic polyester comacromers. Because of the carbon-carbon double bond of fumaric
Min Wang et al.
Acta biomaterialia, 54, 69-80 (2017-02-22)
Development of biodegradable and biocompatible non-viral vectors with intrinsical multifunctional properties such as bioimaging ability for highly efficient nucleic acids delivery still remains a challenge. Here, a biodegradable poly (1,8-octanedio-citric acid)-co-polyethylene glycol grafted with polyethyleneimine (PEI) (POCG-PEI) polymers with the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service